The application of existing genotoxicity methodologies for grouping of nanomaterials: towards an integrated approach to testing and assessment
- PMID: 35525968
- PMCID: PMC9080165
- DOI: 10.1186/s12989-022-00476-9
The application of existing genotoxicity methodologies for grouping of nanomaterials: towards an integrated approach to testing and assessment
Abstract
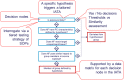
The incorporation of nanomaterials (NMs) in consumer products has proven to be highly valuable in many sectors. Unfortunately, however, the same nano specific physicochemical properties, which make these material attractive, might also contribute to hazards for people exposed to these materials. The physicochemical properties of NMs will impact their interaction with biological surroundings and influence their fate and their potential adverse effects such as genotoxicity. Due to the large and expanding number of NMs produced, their availability in different nanoforms (NFs) and their utilization in various formats, it is impossible for risk assessment to be conducted on an individual NF basis. Alternative methods, such as grouping are needed for streamlining hazard assessment. The GRACIOUS Framework provides a logical and science evidenced approach to group similar NFs, allowing read-across of hazard information from source NFs (or non-NFs) with adequate hazard data to target NFs that lack such data. Here, we propose a simple three-tiered testing strategy to gather evidence to determine whether different NFs are sufficiently similar with respect to their potential to induce genotoxicity, in order to be grouped. The tiered testing strategy includes simple in vitro models as well as a number of alternative more complex multi-cellular in vitro models to allow for a better understanding of secondary NM-induced DNA damage, something that has been more appropriate in vivo until recently.
Keywords: Alternative physiological multi-cellular models; Genotoxicity; Grouping; Nanomaterials; Tiered testing strategy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gkika DA, Vordos N, Magafas L, Mitropoulos AC, Kyzas GZ. Risk return profile of nanomaterials. J Mol Struct. 2021;1228:129740. doi: 10.1016/j.molstruc.2020.129740. - DOI
-
- Belade E, Chrusciel S, Armand L, Simon-Deckers A, Bussy C, Caramelle P, Gagliolo JM, Boyer L, Lanone S, Pairon JC, Kermanizadeh A, Boczkowski J. The role of p53 in lung macrophages following exposure to a panel of manufactured nanomaterials. Arch Toxicol. 2015;89:1543–1556. doi: 10.1007/s00204-014-1324-5. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

