SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: a systematic review and meta-analysis
- PMID: 35525973
- PMCID: PMC9077344
- DOI: 10.1186/s12879-022-07418-y
SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: a systematic review and meta-analysis
Abstract
Background: The temporal evolution of SARS-CoV-2 vaccine efficacy and effectiveness (VE) against infection, symptomatic, and severe COVID-19 is incompletely defined. The temporal evolution of VE could be dependent on age, vaccine types, variants of the virus, and geographic region. We aimed to conduct a systematic review and meta-analysis of the duration of VE against SARS-CoV-2 infection, symptomatic COVID-19 and severe COVID-19.
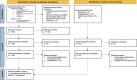
Methods: MEDLINE, Scopus, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, the World Health Organization Global Literature on Coronavirus Disease, and CoronaCentral databases were searched and studies were selected. Independent reviewers selected randomized controlled trials and cohort studies with the outcome of interest. Independent reviewers extracted data, and assessed the risk of bias. Meta-analysis was performed with the DerSimonian-Laird random-effects model with Hartung-Knapp-Sidik-Jonkman variance correction. The GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach was used to assess certainty (quality) of the evidence. Primary outcomes included VE as a function of time against SARS-CoV-2 infection, symptomatic and severe COVID-19.
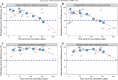
Results: Eighteen studies were included representing nearly 7 million individuals. VE against all SARS-CoV-2 infections declined from 83% in the first month after completion of the original vaccination series to 22% at 5 months or longer. Similarly, VE against symptomatic COVID-19 declined from 94% in the first month after vaccination to 64% by the fourth month. VE against severe COVID-19 for all ages was high overall, with the level being 90% (95% CI, 87-92%) at five months or longer after being fully vaccinated. VE against severe COVID-19 was lower in individuals ≥ 65 years and those who received Ad26.COV2.S.
Conclusions: VE against SARS-CoV-2 infection and symptomatic COVID-19 waned over time but protection remained high against severe COVID-19. These data can be used to inform public health decisions around the need for booster vaccination.
Keywords: COVID-19; Vaccine effectiveness; Waning immunity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Mathieu E, Ritchie H, Ortiz-Ospina E, Roser M, Hasell J, Appel C, Giattino C, Rodés-Guirao L. A global database of COVID-19 vaccinations. Nat Human Behav. 2021;27:1–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

