TRPM8 deficiency attenuates liver fibrosis through S100A9-HNF4α signaling
- PMID: 35525986
- PMCID: PMC9080211
- DOI: 10.1186/s13578-022-00789-4
TRPM8 deficiency attenuates liver fibrosis through S100A9-HNF4α signaling
Abstract
Background: Liver fibrosis represent a major global health care burden. Data emerging from recent advances suggest TRPM8, a member of the transient receptor potential (TRP) family of ion channels, plays an essential role in various chronic inflammatory diseases. However, its role in liver fibrosis remains unknown. Herein, we assessed the potential effect of TRPM8 in liver fibrosis.
Methods: The effect of TRPM8 was evaluated using specimens obtained from classic murine models of liver fibrosis, namely wild-type (WT) and TRPM8-/- (KO) fibrotic mice after carbon tetrachloride (CCl4) or bile duct ligation (BDL) treatment. The role of TRPM8 was systematically evaluated using specimens obtained from the aforementioned animal models after various in vivo and in vitro experiments.
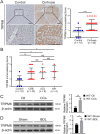
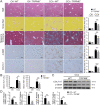
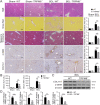
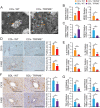
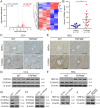
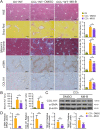
Results: Clinicopathological analysis showed that TRPM8 expression was upregulated in tissue samples from cirrhosis patients and fibrotic mice. TRPM8 deficiency not only attenuated inflammation and fibrosis progression in mice but also helped to alleviate symptoms of cholangiopathies. Moreover, reduction in S100A9 and increase in HNF4α expressions were observed in liver of CCl4- and BDL- treated TRPM8-/- mice. A strong regulatory linkage between S100A9 and HNF4α was also noticed in L02 cells that underwent siRNA-mediated S100A9 knockdown and S100A9 overexpressing plasmid transfection. Lastly, the alleviative effect of a selective TRPM8 antagonist was confirmed in vivo.
Conclusions: These findings suggest TRPM8 deficiency may exert protective effects against inflammation, cholangiopathies, and fibrosis through S100A9-HNF4α signaling. M8-B might be a promising therapeutic candidate for liver fibrosis.
Keywords: ECM; HNF4α; Inflammation; Liver fibrosis; S100A9; TRPM8.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Hic-5 deficiency attenuates the activation of hepatic stellate cells and liver fibrosis through upregulation of Smad7 in mice.J Hepatol. 2016 Jan;64(1):110-7. doi: 10.1016/j.jhep.2015.08.026. Epub 2015 Aug 31. J Hepatol. 2016. PMID: 26334580
-
Functional inhibition of Oct leads to HNF4α upregulation.Exp Ther Med. 2021 Apr;21(4):349. doi: 10.3892/etm.2021.9780. Epub 2021 Feb 11. Exp Ther Med. 2021. PMID: 33732322 Free PMC article.
-
Interleukin-1 Receptor Antagonist Modulates Liver Inflammation and Fibrosis in Mice in a Model-Dependent Manner.Int J Mol Sci. 2019 Mar 14;20(6):1295. doi: 10.3390/ijms20061295. Int J Mol Sci. 2019. PMID: 30875826 Free PMC article.
-
Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis.J Hepatol. 2020 Nov;73(5):1144-1154. doi: 10.1016/j.jhep.2020.04.044. Epub 2020 May 8. J Hepatol. 2020. PMID: 32389810 Free PMC article.
-
Physalin D attenuates hepatic stellate cell activation and liver fibrosis by blocking TGF-β/Smad and YAP signaling.Phytomedicine. 2020 Nov;78:153294. doi: 10.1016/j.phymed.2020.153294. Epub 2020 Jul 28. Phytomedicine. 2020. PMID: 32771890
Cited by
-
Loss of TRPM8 Exacerbate Herpes Simplex Keratitis Infection in Mice by Promoting the Infiltration of CD11b+ Ly6G+ Cells and Increasing the Viral Load in the Cornea.Invest Ophthalmol Vis Sci. 2023 Dec 1;64(15):24. doi: 10.1167/iovs.64.15.24. Invest Ophthalmol Vis Sci. 2023. PMID: 38117245 Free PMC article.
-
TARGETING S100A9-TLR2 AXIS CONTROLS MACROPHAGE NLRP3 INFLAMMASOME ACTIVATION IN FATTY LIVER ISCHEMIA REPERFUSION INJURY.Shock. 2025 Feb 1;63(2):292-298. doi: 10.1097/SHK.0000000000002470. Epub 2024 Oct 21. Shock. 2025. PMID: 39447083 Free PMC article.
-
Programmed Cell Death in Liver Fibrosis.J Inflamm Res. 2023 Sep 1;16:3897-3910. doi: 10.2147/JIR.S427868. eCollection 2023. J Inflamm Res. 2023. PMID: 37674533 Free PMC article. Review.
-
Role of TRP Channels in Liver-Related Diseases.Int J Mol Sci. 2023 Aug 7;24(15):12509. doi: 10.3390/ijms241512509. Int J Mol Sci. 2023. PMID: 37569884 Free PMC article. Review.
-
Multilayer omics reveals the molecular mechanism of early infection of Clonorchis sinensis juvenile.Parasit Vectors. 2023 Aug 16;16(1):285. doi: 10.1186/s13071-023-05891-1. Parasit Vectors. 2023. PMID: 37587524 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

