SARS-CoV-2 vaccine response and rate of breakthrough infection in patients with hematological disorders
- PMID: 35526045
- PMCID: PMC9077637
- DOI: 10.1186/s13045-022-01275-7
SARS-CoV-2 vaccine response and rate of breakthrough infection in patients with hematological disorders
Abstract
Background: The clinical efficacy of SARS-CoV-2 vaccines according to antibody response in immunosuppressed patients such as hematological patients has not yet been established.
Patients and methods: A prospective multicenter registry-based cohort study conducted from December 2020 to December 2021 by the Spanish transplant and cell therapy group was used to analyze the relationship of antibody response at 3-6 weeks after full vaccination (2 doses) with breakthrough SARS-CoV-2 infection in 1394 patients with hematological disorders.
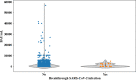
Results: At a median follow-up of 165 days after complete immunization, 37 out of 1394 (2.6%) developed breakthrough SARS-CoV-2 infection at median of 77 days (range 7-195) after full vaccination. The incidence rate was 6.39 per 100 persons-year. Most patients were asymptomatic (19/37, 51.4%), whereas only 19% developed pneumonia. The mortality rate was 8%. Lack of detectable antibodies at 3-6 weeks after full vaccination was the only variable associated with breakthrough infection in multivariate logistic regression analysis (Odds Ratio 2.35, 95% confidence interval 1.2-4.6, p = 0.012). Median antibody titers were lower in cases than in non-cases [1.83 binding antibody units (BAU)/mL (range 0-4854.93) vs 730.81 BAU/mL (range 0-56,800), respectively (p = 0.007)]. We identified 250 BAU/mL as a cutoff above which incidence and severity of the infection were significantly lower.
Conclusions: Our study highlights the benefit of developing an antibody response in these highly immunosuppressed patients. Level of antibody titers at 3 to 6 weeks after 2-dose vaccination links with protection against both breakthrough infection and severe disease for non-Omicron SARS-CoV-2 variants.
Keywords: Allogeneic stem cell transplantation; Autologous stem cell transplantation; Breakthrough SARS-CoV-2 infection; COVID-19; Correlates of protection; Hematological malignancies; Immunocompromised patients; Moderna mRNA-1273; Pfizer-BioNTech BNT162b2; SARS-CoV-2 vaccines; Vaccine.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Piñana JL, Martino R, García-García I, Parody R, Morales MD, Benzo G, et al. Infectious Complications Subcommittee of the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH). Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9:21. doi: 10.1186/s40164-020-00177-z. - DOI - PMC - PubMed
-
- García-Suárez J, de la Cruz J, Cedillo Á, Llamas P, Duarte R, Jiménez-Yuste V, Asociación Madrileña de Hematología y Hemoterapia (AMHH) et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol. 2020;13(1):133. doi: 10.1186/s13045-020-00970-7. - DOI - PMC - PubMed
-
- Pagano L, Salmanton-García J, Marchesi F, Busca A, Corradini P, Hoenigl M, EPICOVIDEHA Working Group et al. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA) J Hematol Oncol. 2021;14(1):168. doi: 10.1186/s13045-021-01177-0. - DOI - PMC - PubMed

