Hypoxic mesenchymal stem cell-derived extracellular vesicles ameliorate renal fibrosis after ischemia-reperfusion injure by restoring CPT1A mediated fatty acid oxidation
- PMID: 35526054
- PMCID: PMC9080148
- DOI: 10.1186/s13287-022-02861-9
Hypoxic mesenchymal stem cell-derived extracellular vesicles ameliorate renal fibrosis after ischemia-reperfusion injure by restoring CPT1A mediated fatty acid oxidation
Abstract
Background: Renal fibrosis is a common pathological process of chronic kidney diseases induced by multiple factors. Hypoxic pretreatment of mesenchymal stem cells can enhance the efficacy of secreted extracellular vesicles (MSC-EVs) on various diseases, but it is not clear whether they can better improve renal fibrosis. The latest research showed that recovery of fatty acid oxidation (FAO) can reduce renal fibrosis. In this study, we aimed to examine whether hypoxic pretreatment with MSC extracellular vesicles (Hypo-EVs) can improve FAO to restore renal fibrosis and to investigate the underlying mechanism.
Methods: Hypo-EVs were isolated from hypoxia-pretreated human placenta-derived MSC (hP-MSC), and Norm-EVs were isolated from hP-MSC cultured under normal conditions. We used ischemia-reperfusion (I/R)-induced renal fibrosis model in vivo. The mice were injected with PBS, Hypo-EVs, or Norm-EVs immediately after the surgery and day 1 postsurgery. Renal function, kidney pathology, and renal fibrosis were assessed for kidney damage evaluation. For mechanistic exploration, fatty acid oxidation (FAO), mitochondrial morphological alterations, ATP production and mitochondrial mass proteins were detected in vivo. Mitochondrial membrane potential and reactive oxygen species (ROS) production were investigated in vitro.
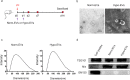
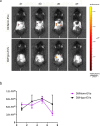
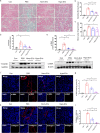
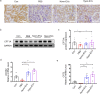
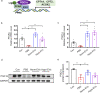
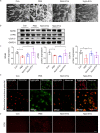
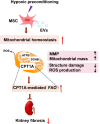
Results: We found that Hypo-EVs confer a superior therapeutic effect on recovery of renal structure damage, restoration of renal function and reduction in renal fibrosis. Meanwhile, Hypo-EVs enhanced mitochondrial FAO in kidney by restoring the expression of a FAO key rate-limiting enzyme carnitine palmitoyl-transferase 1A (CPT1A). Mechanistically, the improvement of mitochondrial homeostasis, characterized by repaired mitochondrial structure, restoration of mitochondrial mass and ATP production, inhibition of oxidative stress, and increased mitochondrial membrane potential, partially explains the effect of Hypo-EVs on improving mitochondrial FAO and thus attenuating I/R damage.
Conclusions: Hypo-EVs suppress the renal fibrosis by restoring CPT1A-mediated mitochondrial FAO, which effects may be achieved through regulation of mitochondrial homeostasis. Our findings provide further mechanism support for development cell-free therapy of renal fibrosis.
Keywords: Extracellular vesicles; Fatty acid oxidation; Hypoxic; Mesenchymal stem cell; Mitochondrial; Renal fibrosis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, Maddukuri G, Tsai CY, Floyd T, Al-Aly Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94(3):567–581. doi: 10.1016/j.kint.2018.04.011. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

