Mesh-like electrospun membrane loaded with atorvastatin facilitates cutaneous wound healing by promoting the paracrine function of mesenchymal stem cells
- PMID: 35526075
- PMCID: PMC9080129
- DOI: 10.1186/s13287-022-02865-5
Mesh-like electrospun membrane loaded with atorvastatin facilitates cutaneous wound healing by promoting the paracrine function of mesenchymal stem cells
Abstract
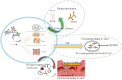
Background: Functional electrospun membranes are promising dressings for promoting wound healing. However, their microstructure and drug loading capacity need further improvements. It is the first time to design a novel mesh-like electrospun fiber loaded with atorvastatin (ATV) and investigated its effects on paracrine secretion by bone marrow-derived mesenchymal stem cells (BMSCs) and wound healing in vivo.
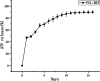
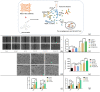
Methods: We fabricated a mesh-like electrospun membrane using a copper mesh receiver. The physical properties of the membranes were evaluated by SEM, FTIR spectroscopy, tensile strength analysis, and contrast angle test. Drug release was measured by plotting concentration as a function of time. We tested the effects of conditioned media (CM) derived from BMSCs on endothelial cell migration and angiogenesis. We used these BMSCs and performed RT-PCR and ELISA to evaluate the expressions of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (b-FGF) genes and proteins, respectively. The involvement of FAK and AKT mechanotransduction pathways in the regulation of BMSC secretion by material surface topography was also investigated. Furthermore, we established a rat model of wound healing, applied ATV-loaded mesh-like membranes (PCL/MAT) seeded with BMSCs on wounds, and assessed their efficacy for promoting wound healing.
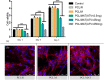
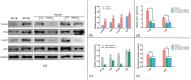
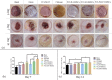
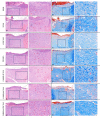
Results: FTIR spectroscopy revealed successful ATV loading in PCL/MAT. Compared with random electrospun fibers (PCL/R) and mesh-like electrospun fibers without drug load (PCL/M), PCL/MAT induced maximum promotion of human umbilical vein endothelial cell (HUVEC) migration. In the PCL/MAT group, the cell sheet scratches were nearly closed after 24 h. However, the cell sheet scratches remained open in other treatments at the same time point. The PCL/MAT promoted angiogenesis and led to the generation of longer tubes than the other treatments. Finally, the PCL/MAT induced maximum gene expression and protein secretion of VEGF and b-FGF. As for material surface topography effect on BMSCs, FAK and AKT signaling pathways were shown to participate in the modulation of MSC morphology and its paracrine function. In vivo, PCL/MAT seeded with BMSCs significantly accelerated healing and improved neovascularization and collagen reconstruction in the wound area compared to the other treatments.
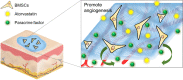
Conclusions: The mesh-like topography of fibrous scaffolds combined with ATV release creates a unique microenvironment that promotes paracrine secretion of BMSCs, thereby accelerating wound healing. Hence, drug-loaded mesh-like electrospun membranes may be highly efficacious for wound healing and as artificial skin. It is a promising approach to solve the traumatic skin defect and accelerate recovery, which is essential to developing functional materials for future regenerative medicine.
Keywords: Atorvastatin; Bone marrow stem cells; Electrospun fibrous; Mesh-like topography; Paracrine secretion; Tissue engineering; Wound healing.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway.Stem Cell Res Ther. 2020 Aug 12;11(1):350. doi: 10.1186/s13287-020-01824-2. Stem Cell Res Ther. 2020. PMID: 32787917 Free PMC article.
-
Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction.Biomaterials. 2017 Oct;141:74-85. doi: 10.1016/j.biomaterials.2017.06.028. Epub 2017 Jun 23. Biomaterials. 2017. PMID: 28667901
-
Autocrine and Paracrine Effects of Vascular Endothelial Cells Promote Cutaneous Wound Healing.Biomed Res Int. 2021 Apr 10;2021:6695663. doi: 10.1155/2021/6695663. eCollection 2021. Biomed Res Int. 2021. PMID: 33937411 Free PMC article.
-
Mesenchymal Stem Cell Migration and Tissue Repair.Cells. 2019 Jul 28;8(8):784. doi: 10.3390/cells8080784. Cells. 2019. PMID: 31357692 Free PMC article. Review.
-
Stem cell-mediated angiogenesis in skin tissue engineering and wound healing.Wound Repair Regen. 2022 Jul;30(4):421-435. doi: 10.1111/wrr.13033. Epub 2022 Jun 14. Wound Repair Regen. 2022. PMID: 35638710 Free PMC article. Review.
Cited by
-
One Molecule, Many Faces: Repositioning Cardiovascular Agents for Advanced Wound Healing.Molecules. 2024 Jun 20;29(12):2938. doi: 10.3390/molecules29122938. Molecules. 2024. PMID: 38931002 Free PMC article. Review.
-
Organoids and Their Research Progress in Plastic and Reconstructive Surgery.Aesthetic Plast Surg. 2023 Apr;47(2):880-891. doi: 10.1007/s00266-022-03129-6. Epub 2022 Nov 18. Aesthetic Plast Surg. 2023. PMID: 36401134 Review.
-
Phytoconstituent-Loaded Nanofibrous Meshes as Wound Dressings: A Concise Review.Pharmaceutics. 2023 Mar 24;15(4):1058. doi: 10.3390/pharmaceutics15041058. Pharmaceutics. 2023. PMID: 37111544 Free PMC article. Review.
-
Translational Challenges in Drug Therapy and Delivery Systems for Treating Chronic Lower Extremity Wounds.Pharmaceutics. 2024 Jun 2;16(6):750. doi: 10.3390/pharmaceutics16060750. Pharmaceutics. 2024. PMID: 38931872 Free PMC article. Review.
-
Engineering strategies to enhance the research progress of mesenchymal stem cells in wound healing.Stem Cell Res Ther. 2025 Jul 1;16(1):342. doi: 10.1186/s13287-025-04471-7. Stem Cell Res Ther. 2025. PMID: 40598499 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

