Taurine and its transporter TAUT positively affect male reproduction and early embryo development
- PMID: 35526154
- PMCID: PMC9156853
- DOI: 10.1093/humrep/deac089
Taurine and its transporter TAUT positively affect male reproduction and early embryo development
Abstract
Study question: Are taurine and its transporter TAUT associated with spermiogenesis and early embryo development?
Summary answer: Morphologically abnormal spermatozoa increased after local functional interference by intratesticular injection, and taurine depletion significantly reduced the normal embryo numbers in vivo and blastocyst formation rate in vitro.
What is known already: Taurine is one of the most abundant amino acids in the male reproductive system and it has been demonstrated that taurine can efficiently improve spermatogenic function in rat models of testicular injury. However, limited information is known about the role of taurine and its transporter TAUT in spermatogenesis and early embryo development.
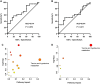
Study design, size, duration: Clinical characteristics from 110 couples who have experienced recurrent pregnancy loss (RPL) were collected from December 2014 to March 2018. According to whether a fetal heartbeat was seen in the previous pregnancy under ultrasonic monitoring, patients with RPL were divided into two groups: an RPL without heartbeat (pregnancy with no fetal heartbeat, ROH) group, and an RPL with heartbeat (one or more pregnancies with fetal heartbeat, RWH) group. Semen samples (21 ROH and 20 RWH) were finally used for metabolomic analysis. Furthermore, semen samples were obtained from 30 patients with teratozoospermia (normal sperm morphology <4%) seeking evaluation for infertility and 25 age-matched control subjects with normal semen quality for western blotting. Animal experiments were performed in CD-1/ICR mice.
Participants/materials, setting, methods: Metabolomics was performed to determine the metabolic changes between the ROH and RWH groups. Sperm proteins from patients with teratozoospermia and healthy controls were extracted for detecting TAUT expression using western blot analysis. Immunofluorescence was used to characterize the localization of TAUT in the testis and ejaculated spermatozoa. Functional analysis in mice was performed by intratesticular injection of siRNAs or antagonist (β-alanine) and 5% β-alanine was provided in drinking water to 3-week-old male mice for 5 weeks with the aim of depleting taurine. Murine epididymal spermatozoa were stained with hematoxylin and eosin for morphological assessment. IVF and mating tests were performed in mice for assessing fertility.
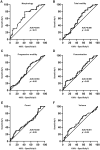
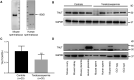
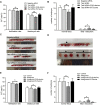
Main results and the role of chance: Metabolomic analysis demonstrated that the taurine content was lower in spermatozoa but higher in seminal plasma from the ROH than the RWH group. TAUT expression was lower in spermatozoa from patients with teratozoospermia than controls. Immunofluorescence showed that TAUT was localized to the manchette in mouse elongated spermatids functional analysis showed that morphologically abnormal spermatozoa increased after interference, and this defect increased after supplementation with 5% β-alanine but was improved by 5% taurine supplementation. Supplementation with 5% β-alanine significantly reduced the normal embryo number in the mouse uterus as well as blastocyst formation rate in vitro.
Large scale data: N/A.
Limitations, reasons for caution: The sample size was low and larger cohorts are needed to confirm the positive effect of taurine on human sperm quality. A comprehensive safety examination should be performed to evaluate whether taurine is a possible treatment for teratozoospermia. Furthermore, the specific molecular mechanism of TAUT involvement in spermiogenesis remains to be clarified.
Wider implications of the findings: The study provides new insights into the role of taurine and its transporter TAUT in male reproduction and embryo development. The results also indicate that TAUT is a promising molecular candidate for the assessment of sperm quality, which may contribute to the diagnosis and treatment for teratozoospermia.
Study funding/competing interest(s): This work was supported by grants from the National Natural Science Foundation of China (no. 81774075, 31900605, 81971451), Jiangsu Science and Technology Program Grant (BK20190654) and Maternal and child health scientific research of Jiangsu Province (F202121). The authors declare no competing financial interests.
Keywords: embryo development; recurrent pregnancy loss; spermiogenesis; taurine; taurine transporter; teratozoospermia.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures



Similar articles
-
Detection of chromosome aberrations in 17 054 individuals with fertility problems and their subsequent assisted reproductive technology treatments in Central China.Hum Reprod. 2023 Nov 20;38(Suppl 2):ii34-ii46. doi: 10.1093/humrep/dead076. Hum Reprod. 2023. PMID: 37982417
-
The influence of male HBV infection on sperm quality, embryonic development, and assisted reproductive outcomes.Hum Reprod. 2024 Jan 5;39(1):43-52. doi: 10.1093/humrep/dead235. Hum Reprod. 2024. PMID: 37994690
-
Characteristics of testis-specific phosphoglycerate kinase 2 and its association with human sperm quality.Hum Reprod. 2016 Feb;31(2):273-9. doi: 10.1093/humrep/dev301. Epub 2015 Dec 17. Hum Reprod. 2016. PMID: 26677959
-
Contribution of semen to early embryo development: fertilization and beyond.Hum Reprod Update. 2023 Jul 5;29(4):395-433. doi: 10.1093/humupd/dmad006. Hum Reprod Update. 2023. PMID: 36882116 Review.
-
Isolated teratozoospermia: revisiting its relevance in male infertility: a narrative review.Transl Androl Urol. 2024 Feb 29;13(2):260-273. doi: 10.21037/tau-23-397. Epub 2024 Feb 26. Transl Androl Urol. 2024. PMID: 38481866 Free PMC article. Review.
Cited by
-
Seminal plasma metabolomics and sperm lipidomics profiles of bull semen with different total progressive motile sperm count.J Anim Sci. 2025 Jan 4;103:skaf012. doi: 10.1093/jas/skaf012. J Anim Sci. 2025. PMID: 39887007
-
The role of taurine in male reproduction: Physiology, pathology and toxicology.Front Endocrinol (Lausanne). 2023 Jan 18;14:1017886. doi: 10.3389/fendo.2023.1017886. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36742382 Free PMC article. Review.
-
TTBK2 affects sperm quality by regulating the expression of centrosomal proteins and flagellar transporters during spermiogenesis in mice.Mol Hum Reprod. 2025 Jul 3;31(3):gaaf030. doi: 10.1093/molehr/gaaf030. Mol Hum Reprod. 2025. PMID: 40581359 Free PMC article.
-
Multigenerational paternal obesity enhances the susceptibility to male subfertility in offspring via Wt1 N6-methyladenosine modification.Nat Commun. 2024 Feb 14;15(1):1353. doi: 10.1038/s41467-024-45675-4. Nat Commun. 2024. PMID: 38355624 Free PMC article.
-
Molecular mechanisms of mammalian sperm capacitation, and its regulation by sodium-dependent secondary active transporters.Reprod Med Biol. 2024 Oct 16;23(1):e12614. doi: 10.1002/rmb2.12614. eCollection 2024 Jan-Dec. Reprod Med Biol. 2024. PMID: 39416520 Free PMC article. Review.
References
-
- Abd-Elhakim YM, Ghoneim MH, Ebraheim LLM, Imam TS. Taurine and hesperidin rescues carbon tetrachloride-triggered testicular and kidney damage in rats via modulating oxidative stress and inflammation. Life Sci 2020;254:117782. - PubMed
-
- Adedara IA, Alake SE, Adeyemo MO, Olajide LO, Ajibade TO, Farombi EO. Taurine enhances spermatogenic function and antioxidant defense mechanisms in testes and epididymis of L-NAME-induced hypertensive rats. Biomed Pharmacother 2018;97:181–189. - PubMed
-
- Baek YY, Cho DH, Choe J, Lee H, Jeoung D, Ha KS, Won MH, Kwon YG, Kim YM. Extracellular taurine induces angiogenesis by activating ERK-, Akt-, and FAK-dependent signal pathways. Eur J Pharmacol 2012;674:188–199. - PubMed
-
- Boguenet M, Bocca C, Bouet PE, Serri O, Chupin S, Tessier L, Blanchet O, El Hachem H, Chao de la Barca JM, Reynier P et al. Metabolomic signature of the seminal plasma in men with severe oligoasthenospermia. Andrologia 2020;8:1859–1866. - PubMed
-
- Brugnon F, Ouchchane L, Pons-Rejraji H, Artonne C, Farigoule M, Janny L. Density gradient centrifugation prior to cryopreservation and hypotaurine supplementation improve post-thaw quality of sperm from infertile men with oligoasthenoteratozoospermia. Hum Reprod 2013;28:2045–2057. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

