Prognostic value of integrin αV expression and localization pattern in invasive breast carcinomas
- PMID: 35526305
- PMCID: PMC9092997
- DOI: 10.1016/j.neo.2022.100803
Prognostic value of integrin αV expression and localization pattern in invasive breast carcinomas
Abstract
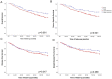
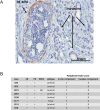
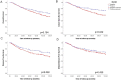
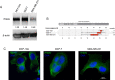
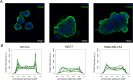
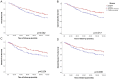
Invasion of surrounding stroma is an early event in breast cancer metastatic progression, and involves loss of cell polarity, loss of myoepithelial layer, epithelial-mesenchymal transition (EMT) and remodeling of the extracellular matrix (ECM). Integrins are transmembrane receptors responsible for cell-ECM binding, which triggers signals that regulate many aspects of cell behavior and fate. Changes in the expression, localization and pairing of integrins contribute for abnormal responses found in transformed epithelia. We analyzed 345 human breast cancer samples in tissue microarrays (TMA) from cases diagnosed with invasive breast carcinoma to assess the expression and localization pattern of integrin αV and correlation with clinical parameters. Patients with lower levels of integrin αV staining showed reduced cancer specific survival. A subset of cases presented a peripheral staining of integrin αV surrounding tumor cell clusters, possibly matching the remaining myoepithelial layer. Indeed, the majority of ductal carcinoma in situ (DCIS) components found in the TMA presented integrin αV at their periphery, whereas this pattern was mostly lost in invasive components, even in the same sample. The lack of peripheral integrin αV correlated with decreased cancer specific survival. In addition, we observed that the presence of integrin αV in the stroma was an indicative of poor survival and metastatic disease. Consistently, by interrogating publicly available datasets we found that, although patients with higher mRNA levels of integrin αV had increased risk of developing metastasis, high co-expression of integrin αV and a myoepithelial cell marker (MYH11) mRNA levels correlated with better clinical outcomes. Finally, a 3D cell culture model of non-malignant and malignant cells reproduced the integrin αV pattern seen in patient samples. Taken together, our data indicate that both the expression levels of integrin αV and its tissue localization in primary tumors have prognostic value, and thus, could be used to help predict patients at higher risk of developing metastasis.
Keywords: 3D cell culture; Breast cancer; Integrin αV; Metastasis; Tissue microarray; Tumor stroma.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Conflict of interests The authors declare no conflict of interest
Figures


Similar articles
-
Genetic depletion and pharmacological targeting of αv integrin in breast cancer cells impairs metastasis in zebrafish and mouse xenograft models.Breast Cancer Res. 2015 Feb 25;17(1):28. doi: 10.1186/s13058-015-0537-8. Breast Cancer Res. 2015. PMID: 25849225 Free PMC article.
-
αv-Integrin isoform expression in primary human tumors and brain metastases.Int J Cancer. 2013 Nov 15;133(10):2362-71. doi: 10.1002/ijc.28267. Epub 2013 Jun 10. Int J Cancer. 2013. PMID: 23661241
-
Interactions between αv-Integrin and HER2 and Their Role in the Invasive Phenotype of Breast Cancer Cells In Vitro and in Rat Brain.PLoS One. 2015 Jul 29;10(7):e0131842. doi: 10.1371/journal.pone.0131842. eCollection 2015. PLoS One. 2015. PMID: 26222911 Free PMC article.
-
Overexpression of integrin αv in the human nasopharyngeal carcinoma associated with metastasis and progression.Cancer Biomark. 2013;13(5):323-8. doi: 10.3233/CBM-130361. Cancer Biomark. 2013. PMID: 24440971
-
αV integrins in angiogenesis and cancer.Cold Spring Harb Perspect Med. 2011 Sep;1(1):a006478. doi: 10.1101/cshperspect.a006478. Cold Spring Harb Perspect Med. 2011. PMID: 22229119 Free PMC article. Review.
Cited by
-
Prognostic value of Maspin protein level in patients with triple negative breast cancer.Sci Rep. 2024 Jul 10;14(1):15982. doi: 10.1038/s41598-024-53870-y. Sci Rep. 2024. PMID: 38987610 Free PMC article.
-
MCL1 Inhibition Overcomes the Aggressiveness Features of Triple-Negative Breast Cancer MDA-MB-231 Cells.Int J Mol Sci. 2023 Jul 6;24(13):11149. doi: 10.3390/ijms241311149. Int J Mol Sci. 2023. PMID: 37446326 Free PMC article.
References
-
- Weigelt B., Peterse J.L., van't Veer L.J. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5(8):591–602. - PubMed
-
- Gumbiner B.M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84(3):345–357. - PubMed
-
- Van der Flier A., Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305(3):285–298. - PubMed
-
- Geiger B., Bershadsky A. Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell. 2002;110(2):139–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

