Safety profile of hydroxychloroquine used off-label for the treatment of patients with COVID-19: A descriptive study based on EudraVigilance data
- PMID: 35526987
- PMCID: PMC9348099
- DOI: 10.1111/fcp.12797
Safety profile of hydroxychloroquine used off-label for the treatment of patients with COVID-19: A descriptive study based on EudraVigilance data
Abstract
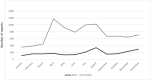
At the beginning of the COVID-19 pandemic, worldwide attempts were made to identify potential drugs effective against the COVID-19. Hydroxychloroquine was among the first receiving attention. However, following its use in therapy, it has been shown that hydroxychloroquine was not only ineffective but probably, due to its known side effects, even responsible of increased mortality of patients. The objective of this study was to review the safety profile of hydroxychloroquine used off-label for the treatment of COVID-19. We analyze the reports of suspected adverse drug reactions (ADRs) collected in EudraVigilance, the European database of ADR reports. We collected 2266 reports for 2019 and 6525 for 2020. The most reported ADRs during 2020 were those relating to cardiac, hepatic, renal toxicity such as QT prolongation with 400 cases in 2020 (of which, 345 cases-9.97%-with COVID-19 as a therapeutic indication) versus 1 case only in 2019 (0.01%), long QT syndrome: 38 cases in 2020 (36 as COVID-19 treatment) versus 0 in 2019, hepatitis: 13 cases in 2019 (0.11%) and 132 in 2020, and 32 cases (24, 0.69%) of acute kidney injury in 2020 and only 3 cases in 2019. Moreover, some important vision-related ADRs also increased significantly during 2020, such as retinal toxicity with 92 cases in 2020 versus 7 in 2019. Even though with its intrinsic limitations, our results may be added to the most recent scientific evidence to confirm the unfavorable risk profile of hydroxychloroquine in its off-label use in the treatment of COVID-19 disease.
Keywords: COVID-19; benefit-risk balance; hydroxychloroquine; off-label; safety.
© 2022 The Authors. Fundamental & Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of Société Française de Pharmacologie et de Thérapeutique.
Conflict of interest statement
All the authors declare no conflict of interest
Figures
Similar articles
-
"Off-label" use of hydroxychloroquine, azithromycin, lopinavir-ritonavir and chloroquine in COVID-19: A survey of cardiac adverse drug reactions by the French Network of Pharmacovigilance Centers.Therapie. 2020 Jul-Aug;75(4):371-379. doi: 10.1016/j.therap.2020.05.002. Epub 2020 May 7. Therapie. 2020. PMID: 32418730 Free PMC article.
-
Off-Label Use of Hydroxychloroquine in COVID-19: Analysis of Reports of Suspected Adverse Reactions From the Italian National Network of Pharmacovigilance.J Clin Pharmacol. 2022 May;62(5):646-655. doi: 10.1002/jcph.2006. Epub 2022 Jan 5. J Clin Pharmacol. 2022. PMID: 34802170 Free PMC article.
-
Comparative study of the adverse event profile of hydroxychloroquine before and during the Sars-CoV2 pandemic.Therapie. 2022 May-Jun;77(3):301-307. doi: 10.1016/j.therap.2021.12.015. Epub 2021 Dec 28. Therapie. 2022. PMID: 35568573 Free PMC article.
-
Off-label use of chloroquine, hydroxychloroquine, azithromycin and lopinavir/ritonavir in COVID-19 risks prolonging the QT interval by targeting the hERG channel.Eur J Pharmacol. 2021 Feb 15;893:173813. doi: 10.1016/j.ejphar.2020.173813. Epub 2020 Dec 18. Eur J Pharmacol. 2021. PMID: 33345848 Free PMC article. Review.
-
QTc prolongation in COVID-19 patients treated with hydroxychloroquine, chloroquine, azithromycin, or lopinavir/ritonavir: A systematic review and meta-analysis.Pharmacoepidemiol Drug Saf. 2021 Jun;30(6):694-706. doi: 10.1002/pds.5234. Epub 2021 Apr 3. Pharmacoepidemiol Drug Saf. 2021. PMID: 33772933 Free PMC article.
Cited by
-
Managing Traumatic Brain Injury During the Coronavirus Disease 2019 Pandemic-A Case-Matched Controlled Analysis of Immediate Outcomes.World Neurosurg. 2022 Sep;165:e59-e73. doi: 10.1016/j.wneu.2022.05.076. Epub 2022 May 25. World Neurosurg. 2022. PMID: 35643408 Free PMC article.
-
[Central nervous system adverse events potentially associated with drugs used for COVID-19: scoping reviewEventos adversos en el sistema nervioso central potencialmente relacionados con los medicamentos para tratar la COVID-19: revisión exploratoria].Rev Panam Salud Publica. 2022 Oct 25;46:e166. doi: 10.26633/RPSP.2022.166. eCollection 2022. Rev Panam Salud Publica. 2022. PMID: 36320207 Free PMC article. Portuguese.
-
Comparative Analyses of the Safety Profiles of Vitamin D Receptor Agonists: A Pharmacovigilance Study Based on the EudraVigilance Database.Pharmaceuticals (Basel). 2024 Dec 13;17(12):1686. doi: 10.3390/ph17121686. Pharmaceuticals (Basel). 2024. PMID: 39770528 Free PMC article.
-
The use and safety risk of repurposed drugs for COVID-19 patients: lessons learned utilizing the Food and Drug Administration's Adverse Event Reporting System.Saudi Pharm J. 2023 Jul;31(7):1360-1366. doi: 10.1016/j.jsps.2023.05.023. Epub 2023 Jun 1. Saudi Pharm J. 2023. PMID: 37304358 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials