Nasal delivery of broadly neutralizing antibodies protects mice from lethal challenge with SARS-CoV-2 delta and omicron variants
- PMID: 35527227
- PMCID: PMC8855614
- DOI: 10.1016/j.virs.2022.02.005
Nasal delivery of broadly neutralizing antibodies protects mice from lethal challenge with SARS-CoV-2 delta and omicron variants
Abstract
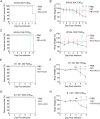
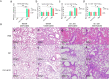
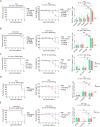
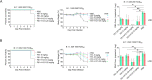
Multiple new variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have constantly emerged, as the delta and omicron variants, which have developed resistance to currently gained neutralizing antibodies. This highlights a critical need to discover new therapeutic agents to overcome the variants mutations. Despite the availability of vaccines against coronavirus disease 2019 (COVID-19), the use of broadly neutralizing antibodies has been considered as an alternative way for the prevention or treatment of SARS-CoV-2 variants infection. Here, we show that the nasal delivery of two previously characterized broadly neutralizing antibodies (F61 and H121) protected K18-hACE2 mice against lethal challenge with SARS-CoV-2 variants. The broadly protective efficacy of the F61 or F61/F121 cocktail antibodies was evaluated by lethal challenge with the wild strain (WIV04) and multiple variants, including beta (B.1.351), delta (B.1.617.2), and omicron (B.1.1.529) at 200 or 1000 TCID50, and the minimum antibody administration doses (5-1.25 mg/kg body weight) were also evaluated with delta and omicron challenge. Fully prophylactic protections were found in all challenged groups with both F61 and F61/H121 combination at the administration dose of 20 mg/kg body weight, and corresponding mice lung viral RNA showed negative, with almost all alveolar septa and cavities remaining normal. Furthermore, low-dose antibody treatment induced significant prophylactic protection against lethal challenge with delta and omicron variants, whereas the F61/H121 combination showed excellent results against omicron infection. Our findings indicated the potential use of broadly neutralizing monoclonal antibodies as prophylactic and therapeutic agent for protection of current emerged SARS-CoV-2 variants infection.
Keywords: Coronavirus disease 2019 (COVID-2019); K18-hACE2; Omicron variant; Prophylactic protection; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Copyright © 2022 The Authors. Publishing services by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Structural basis of a two-antibody cocktail exhibiting highly potent and broadly neutralizing activities against SARS-CoV-2 variants including diverse Omicron sublineages.Cell Discov. 2022 Sep 8;8(1):87. doi: 10.1038/s41421-022-00449-4. Cell Discov. 2022. PMID: 36075908 Free PMC article.
-
A Glycosylated RBD Protein Induces Enhanced Neutralizing Antibodies against Omicron and Other Variants with Improved Protection against SARS-CoV-2 Infection.J Virol. 2022 Sep 14;96(17):e0011822. doi: 10.1128/jvi.00118-22. Epub 2022 Aug 16. J Virol. 2022. PMID: 35972290 Free PMC article.
-
Broadly neutralizing antibodies against Omicron-included SARS-CoV-2 variants induced by vaccination.Signal Transduct Target Ther. 2022 Apr 27;7(1):139. doi: 10.1038/s41392-022-00987-z. Signal Transduct Target Ther. 2022. PMID: 35478188 Free PMC article.
-
Transchromosomic bovines-derived broadly neutralizing antibodies as potent biotherapeutics to counter important emerging viral pathogens with a special focus on SARS-CoV-2, MERS-CoV, Ebola, Zika, HIV-1, and influenza A virus.J Med Virol. 2022 Oct;94(10):4599-4610. doi: 10.1002/jmv.27907. Epub 2022 Jun 11. J Med Virol. 2022. PMID: 35655326 Free PMC article. Review.
-
Broad strategies for neutralizing SARS-CoV-2 and other human coronaviruses with monoclonal antibodies.Sci China Life Sci. 2023 Apr;66(4):658-678. doi: 10.1007/s11427-022-2215-6. Epub 2022 Nov 24. Sci China Life Sci. 2023. PMID: 36443513 Free PMC article. Review.
Cited by
-
Functional nucleic acids as potent therapeutics against SARS-CoV-2 infection.Cell Rep Phys Sci. 2023 Feb 15;4(2):101249. doi: 10.1016/j.xcrp.2023.101249. Epub 2023 Jan 23. Cell Rep Phys Sci. 2023. PMID: 36714073 Free PMC article. Review.
-
Passive antibody therapy in emerging infectious diseases.Front Med. 2023 Dec;17(6):1117-1134. doi: 10.1007/s11684-023-1021-y. Epub 2023 Dec 2. Front Med. 2023. PMID: 38040914 Review.
-
Human monoclonal antibody F61 nasal spray effectively protected high-risk populations from SARS-CoV-2 variants during the COVID-19 pandemic from late 2022 to early 2023 in China.Emerg Microbes Infect. 2024 Dec;13(1):2284297. doi: 10.1080/22221751.2023.2284297. Epub 2024 Mar 26. Emerg Microbes Infect. 2024. PMID: 37970736 Free PMC article. Clinical Trial.
-
Structural basis of a two-antibody cocktail exhibiting highly potent and broadly neutralizing activities against SARS-CoV-2 variants including diverse Omicron sublineages.Cell Discov. 2022 Sep 8;8(1):87. doi: 10.1038/s41421-022-00449-4. Cell Discov. 2022. PMID: 36075908 Free PMC article.
-
COVID-19 Therapeutic Potential of Natural Products.Int J Mol Sci. 2023 May 31;24(11):9589. doi: 10.3390/ijms24119589. Int J Mol Sci. 2023. PMID: 37298539 Free PMC article. Review.
References
-
- Barnes C.O., Jette C.A., Abernathy M.E., Dam K.A., Esswein S.R., Gristick H.B., Malyutin A.G., Sharaf N.G., Huey-Tubman K.E., Lee Y.E., Robbiani D.F., Nussenzweig M.C., West A.P., Jr., Bjorkman P.J. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588:682–687. - PMC - PubMed
-
- Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature. 2021;600:21. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

