Effects of long-acting muscarinic antagonists on promoting ciliary function in airway epithelium
- PMID: 35527239
- PMCID: PMC9080152
- DOI: 10.1186/s12890-022-01983-3
Effects of long-acting muscarinic antagonists on promoting ciliary function in airway epithelium
Abstract
Background: Mucociliary clearance (MCC) is an essential defense mechanism in airway epithelia for removing pathogens from the respiratory tract. Impaired ciliary functions and MCC have been demonstrated in asthma and chronic obstructive pulmonary disease (COPD). Long-acting muscarinic antagonists (LAMAs) are a major class of inhaled bronchodilators, which are used for treating asthma and COPD; however, the effects of LAMAs on ciliary function remain unclear. This study aimed to identify the effects of LAMAs on airway ciliary functions.
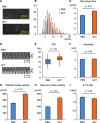
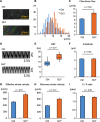
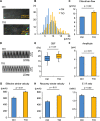
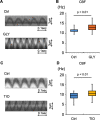
Methods: Wild-type BALB/c mice were treated with daily intranasal administrations of glycopyrronium for 7 days, and tracheal samples were collected. Cilia-driven flow and ciliary activity, including ciliary beat frequency (CBF), ciliary beating amplitude, effective stroke velocity, recovery stroke velocity and the ratio of effective stroke velocity to recovery stroke velocity, were analyzed by imaging techniques. Using in vitro murine models, tracheal tissues were transiently cultured in media with/without LAMAs, glycopyrronium or tiotropium, for 60 min. Cilia-driven flow and ciliary activity were then analyzed. Well-differentiated normal human bronchial epithelial (NHBE) cells were treated with glycopyrronium, tiotropium, or vehicle for 60 min, and CBF was evaluated. Several mechanistic analyses were performed.
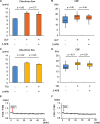
Results: Intranasal glycopyrronium administration for 7 days significantly increased cilia-driven flow and ciliary activity in murine airway epithelium. In the murine tracheal organ culture models, treatment with glycopyrronium or tiotropium for 60 min significantly increased cilia-driven flow and ciliary activity in airway epithelium. Further, we confirmed that 60-min treatment with glycopyrronium or tiotropium directly increased CBF in well-differentiated NHBE cells. In the mechanistic analyses, neither treatment with glycopyrronium nor tiotropium affected intracellular calcium ion concentrations in well-differentiated NHBE cells. Glycopyrronium did not increase protein kinase A activity in well-differentiated NHBE cells. Moreover, glycopyrronium had no effect on extracellular adenosine triphosphate concentration.
Conclusions: LAMAs exert a direct effect on airway epithelium to enhance ciliary function, which may improve impaired MCC in asthma and COPD. Further investigations are warranted to elucidate the underlying mechanisms of the effects of LAMAs on the promotion of airway ciliary function.
Keywords: Airway epithelium; Asthma; COPD; Ciliary beat frequency; Long-acting muscarinic antagonists; Mucociliary clearance.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interest.
Figures

References
-
- Kusagaya H, Fujisawa T, Yamanaka K, Mori K, Hashimoto D, Enomoto N, Inui N, Nakamura Y, Wu R, Maekawa M, et al. Toll-like receptor-mediated airway IL-17C enhances epithelial host defense in an autocrine/paracrine manner. Am J Respir Cell Mol Biol. 2014;50(1):30–39. - PubMed
-
- Niwa M, Fujisawa T, Mori K, Yamanaka K, Yasui H, Suzuki Y, Karayama M, Hozumi H, Furuhashi K, Enomoto N, et al. IL-17A attenuates IFN-lambda expression by inducing suppressor of cytokine signaling expression in airway epithelium. J Immunol. 2018;201(8):2392–2402. doi: 10.4049/jimmunol.1800147. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

