Time-course changes in DNA damage of corneal epithelial cells in rabbits following ocular instillation with genotoxic compounds
- PMID: 35527291
- PMCID: PMC9082918
- DOI: 10.1186/s41021-022-00243-4
Time-course changes in DNA damage of corneal epithelial cells in rabbits following ocular instillation with genotoxic compounds
Abstract
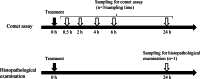
Background: In eye-drop drug development, the additional genotoxicity tests in some cases might be necessary to assess genotoxicity in the ocular surface since the ocular surface is exposed directly to high drug concentrations. Recently, an in vivo comet assay using corneal epithelial cells in rabbits following single ocular instillation was developed as an assay to evaluate genotoxicity in ocular tissues. In this study, we investigated the time-course changes in DNA damage after ocular instillation of genotoxic compounds to evaluate the optimal sampling timing for in vivo comet assay of the ocular surface tissue. Ethidium bromide (EtBr), methyl methanesulfonate (MMS), and 4-nitroquinoline 1-oxide (4-NQO) were administered to the eyes of the rabbits. Corneas were collected at 0.5, 2, 4, 6, and 24 h after administration, and the comet assay was performed. In addition, the in vitro comet assay was performed to assess the time-course changes in DNA damage induced by short-time exposure to the genotoxic compounds.
Results: The mean % tail DNA, which is an indicator for DNA damage, in the corneal epithelial cells treated with all compounds exhibited statistically significant increases compared with those in the negative controls of saline at 0.5, 2, 4, and 6 h. There was a difference in the DNA damage response between EtBr and the other two compounds. In the 3% MMS- and 1% 4-NQO-treated eyes, the values of the % tail DNA were the highest at 0.5 h and then decreased gradually. In contrast, in the 1% EtBr-treated eyes, the highest value was noted at 4 h. The results of the in vitro comet assay showed that the % tail DNA increased in all groups. A further increase in the % tail DNA occurred in the EtBr-treated cells even after removing the compound but not in the MMS- and 4-NQO-treated cells.
Conclusion: Relatively high values of the % tail DNA were maintained from 0.5 to 6 h after administration, suggesting that the optimal sampling time is any one point from 0.5 to 6 h in the comet assay of the corneal surface.
Keywords: Comet assay; Corneal epithelial cells; Genotoxicity test; Ophthalmic drug; Rabbit; Sampling time.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
In vivo comet assay in rabbit corneal epithelial cells following ocular instillation with genotoxic compounds.Genes Environ. 2021 Apr 7;43(1):11. doi: 10.1186/s41021-021-00184-4. Genes Environ. 2021. PMID: 33827709 Free PMC article.
-
Investigation of comet assays under conditions mimicking ocular instillation administration in a three-dimensional reconstructed human corneal epithelial model.Cutan Ocul Toxicol. 2019 Dec;38(4):375-383. doi: 10.1080/15569527.2019.1634580. Epub 2019 Jul 8. Cutan Ocul Toxicol. 2019. PMID: 31223032
-
Investigation of in vivo unscheduled DNA synthesis in rabbit corneas following instillation of genotoxic agents.Cutan Ocul Toxicol. 2021 Mar;40(1):26-36. doi: 10.1080/15569527.2021.1874006. Epub 2021 Jan 18. Cutan Ocul Toxicol. 2021. PMID: 33461361
-
The comet assay with multiple mouse organs: comparison of comet assay results and carcinogenicity with 208 chemicals selected from the IARC monographs and U.S. NTP Carcinogenicity Database.Crit Rev Toxicol. 2000 Nov;30(6):629-799. doi: 10.1080/10408440008951123. Crit Rev Toxicol. 2000. PMID: 11145306 Review.
-
Ocular Cell Lines and Genotoxicity Assessment.Int J Environ Res Public Health. 2020 Mar 19;17(6):2046. doi: 10.3390/ijerph17062046. Int J Environ Res Public Health. 2020. PMID: 32204489 Free PMC article. Review.
References
-
- Kurata M, Atsumi I, Yamagiwa Y, Sakaki H. Ocular instillation toxicity study: current status and points to consider on study design and evaluation. Fundam Toxicol Sci. 2016;3(5):217–232. doi: 10.2131/fts.3.217. - DOI
-
- Organisation for Economic Cooperation and Development. OECD guideline for the testing of chemicals. In vivo mammalian alkaline comet assay (TG 489). Section 4. 2016. p. 1–27.
-
- Kurata M, Yamagiwa Y, Haranosono Y, Sakaki H. Repeated-dose ocular instillation toxicity study: a survey of its study design on the basis of common technical documents in Japan. Fundam Toxicol Sci. 2017;4(2):95–99. doi: 10.2131/fts.4.95. - DOI
LinkOut - more resources
Full Text Sources

