New treatment paradigm with systemic therapy in intermediate-stage hepatocellular carcinoma
- PMID: 35527313
- PMCID: PMC9209396
- DOI: 10.1007/s10147-022-02166-0
New treatment paradigm with systemic therapy in intermediate-stage hepatocellular carcinoma
Abstract
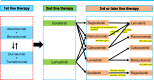
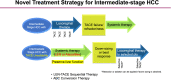
Since the approval of sorafenib for the treatment of unresectable hepatocellular carcinoma in 2007 (in 2009 in Japan), five more regimens have been approved: lenvatinib, and atezolizumab plus bevacizumab for first-line treatment, and regorafenib, cabozantinib, and ramucirumab for second-line treatment, which are currently available for clinical use. The positive results of durvalumab, a programmed cell death ligand 1 antibody, plus tremelimumab, an anti-cytotoxic T-lymphocyte-associated protein 4 antibody, were also presented at the 2022 American Society Clinical Oncology Gastrointestinal Cancers Symposium as superior to sorafenib in prolonging the overall survival; this combination is expected to be approved by the end of 2022. These systemic therapies are changing the treatment paradigm not only for advanced hepatocellular carcinoma but also for intermediate-stage hepatocellular carcinoma. This review focuses on the role of systemic therapy in intermediate-stage hepatocellular carcinoma.
Keywords: Hepatocellular carcinoma; Immune checkpoint inhibitors; Immune microenvironment; Molecular targeted therapy; Systemic therapy.
© 2022. The Author(s).
Conflict of interest statement
Lecture: Eisai, Bayer, MSD, BMS, EA Pharma, Eli Lilly, Chugai; Grants: Eisai, Takeda, Otsuka, Taiho, EA Pharma, Gilead Sciences, Abbvie, Sumitomo Dainippon Pharma, Chugai, Ono Pharma; Advisory Consulting: Eisai, Ono, MSD, BMS, Roche.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

