Generic benzalkonium chloride-preserved travoprost eye drops are not identical to the branded polyquarternium-1-preserved travoprost eye drop: Effect on cultured human conjunctival goblet cells and their physicochemical properties
- PMID: 35527390
- PMCID: PMC9790398
- DOI: 10.1111/aos.15163
Generic benzalkonium chloride-preserved travoprost eye drops are not identical to the branded polyquarternium-1-preserved travoprost eye drop: Effect on cultured human conjunctival goblet cells and their physicochemical properties
Abstract
Purpose: To investigate the effect of polyquaternium-1 (PQ)-preserved and benzalkonium chloride (BAK)-preserved travoprost eye drops on viability of primary human conjunctival goblet cell (GC) cultures and on secretion of mucin and cytokines. Furthermore, to evaluate the physicochemical properties of the branded travoprost eye drop Travatan® and available generics.
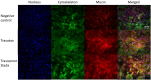
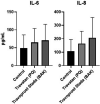
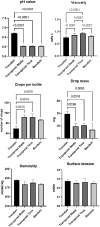
Methods: The effect of travoprost eye drops was evaluated on GC cultures. Cell viability was assessed through lactate dehydrogenase (LDH) and tetrazolium dye (MTT) colorimetric assays. Mucin secretion was evaluated by immunohistochemical staining. Secretion of interleukin (IL)-6 and IL-8 was measured using BD Cytometric Bead Arrays. pH, viscosity, droplet mass, osmolality and surface tension were measured for all included eye drops.
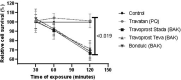
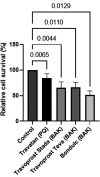
Results: In the LDH assay, BAK travoprost caused significant GC loss after 2 hrs of incubation compared to the control. PQ travoprost caused no GC loss at any time point. Both PQ- and BAK travoprost caused secretion of mucin to the cytoplasma. No difference in IL-6 and IL-8 secretion was identified compared to controls. The pH values for the generics were lower (pH 6.0) than the pH value for Travatan (pH 6.7; p < 0.0001). The viscosity was lowest for Travatan, while the mean droplet mass was higher for Travatan (35 mg) than the generics (28-30 mg; p ≤ 0.0318). The osmolality and surface tension did not differ between the eye drops investigated.
Conclusion: BAK travoprost caused GC loss, indicating that PQ preservation may be preferable in treatment of glaucoma. Furthermore, physicochemical properties of branded and generic travoprost eye drops can not be assumed to be identical.
Keywords: benzalkonium chloride; glaucoma; goblet cells; ocular surface; polyquaternium-1; travoprost.
© 2022 The Authors. Acta Ophthalmologica published by John Wiley & Sons Ltd on behalf of Acta Ophthalmologica Scandinavica Foundation.
Figures
Similar articles
-
Comparing the effect of benzalkonium chloride-preserved, polyquad-preserved, and preservative-free prostaglandin analogue eye drops on cultured human conjunctival goblet cells.J Optom. 2024 Jan-Mar;17(1):100481. doi: 10.1016/j.optom.2023.100481. Epub 2023 Oct 1. J Optom. 2024. PMID: 37788596 Free PMC article.
-
The use of benzalkonium chloride in topical glaucoma treatment: An investigation of the efficacy and safety of benzalkonium chloride-preserved intraocular pressure-lowering eye drops and their effect on conjunctival goblet cells.Acta Ophthalmol. 2023 Dec;101 Suppl 278:3-21. doi: 10.1111/aos.15808. Acta Ophthalmol. 2023. PMID: 38037546
-
The preservative polyquaternium-1 increases cytoxicity and NF-kappaB linked inflammation in human corneal epithelial cells.Mol Vis. 2012;18:1189-96. Epub 2012 May 6. Mol Vis. 2012. PMID: 22605930 Free PMC article.
-
Reduced in vivo ocular surface toxicity with polyquad-preserved travoprost versus benzalkonium-preserved travoprost or latanoprost ophthalmic solutions.Ophthalmic Res. 2012;48(2):89-101. doi: 10.1159/000335984. Ophthalmic Res. 2012. PMID: 22473057
-
Evaluation of Physical Properties of Generic and Branded Travoprost Formulations.J Curr Glaucoma Pract. 2016 May-Aug;10(2):49-55. doi: 10.5005/jp-journals-10008-1201. Epub 2016 Aug 5. J Curr Glaucoma Pract. 2016. PMID: 27536047 Free PMC article. Review.
Cited by
-
Ocular surface disease: a known yet overlooked side effect of topical glaucoma therapy.Front Toxicol. 2023 Jul 21;5:1067942. doi: 10.3389/ftox.2023.1067942. eCollection 2023. Front Toxicol. 2023. PMID: 37547228 Free PMC article. Review.
-
Efficacy and Toxicity Evaluation of Bepotastine Besilate 1.5% Preservative-Free Eye Drops Vs Olopatadine Hydrochloride 0.2% Bak-Preserved Eye Drops in Patients with Allergic Conjunctivitis.Clin Ophthalmol. 2023 Nov 16;17:3477-3489. doi: 10.2147/OPTH.S431889. eCollection 2023. Clin Ophthalmol. 2023. PMID: 38026598 Free PMC article. Clinical Trial.
-
SARS-CoV-2 positivity rates in tear secretions of inpatient COVID-positive individuals in an Indian tertiary care setting.Indian J Ophthalmol. 2025 Feb 1;73(2):273-279. doi: 10.4103/IJO.IJO_2347_23. Epub 2024 Jul 11. Indian J Ophthalmol. 2025. PMID: 38990621 Free PMC article.
-
Can the choice of artificial tears harm patients? A narrative review with an overview of the Nordic market.Acta Ophthalmol. 2025 Aug;103(5):586-608. doi: 10.1111/aos.17455. Epub 2025 Feb 8. Acta Ophthalmol. 2025. PMID: 39921541 Free PMC article. Review.
References
-
- Baudouin C, Rolando M, Benitez Del Castillo JM et al. (2019): Reconsidering the central role of mucins in dry eye and ocular surface diseases. Prog Retin Eye Res 71: 68–87. - PubMed
-
- Brignole‐Baudouin F, Riancho L, Liang H & Baudouin C (2011): Comparative in vitro toxicology study of travoprost polyquad‐preserved, travoprost BAK‐preserved, and latanoprost BAK‐preserved ophthalmic solutions on human conjunctival epithelial cells. Curr Eye Res 36: 979–988. - PubMed
-
- Danish Medicines Agency (2019a): Substitution https://laegemiddelstyrelsen.dk/da/apoteker/substitution/. (Accessed 28 sep 2021).
-
- Danish Medicines Agency (2019b): Product summary for Bondulc.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

