Influence of effective polarization on ion and water interactions within a biomimetic nanopore
- PMID: 35527400
- PMCID: PMC9247480
- DOI: 10.1016/j.bpj.2022.05.006
Influence of effective polarization on ion and water interactions within a biomimetic nanopore
Abstract
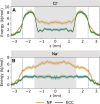
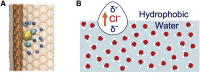
Interactions between ions and water at hydrophobic interfaces within ion channels and nanopores are suggested to play a key role in the movement of ions across biological membranes. Previous molecular-dynamics simulations have shown that anion affinity for aqueous/hydrophobic interfaces can be markedly influenced by including polarization effects through an electronic continuum correction. Here, we designed a model biomimetic nanopore to imitate the polar pore openings and hydrophobic gating regions found in pentameric ligand-gated ion channels. Molecular-dynamics simulations were then performed using both a non-polarizable force field and the electronic-continuum-correction method to investigate the behavior of water, Na+, and Cl- ions confined within the hydrophobic region of the nanopore. Number-density distributions revealed preferential Cl- adsorption to the hydrophobic pore walls, with this interfacial layer largely devoid of Na+. Free-energy profiles for Na+ and Cl- permeating the pore also display an energy-barrier reduction associated with the localization of Cl- to this hydrophobic interface, and the hydration-number profiles reflect a corresponding reduction in the first hydration shell of Cl-. Crucially, these ion effects were only observed through inclusion of effective polarization, which therefore suggests that polarizability may be essential for an accurate description for the behavior of ions and water within hydrophobic nanoscale pores, especially those that conduct Cl-.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
J.C. is an employee of IBM Research. All other authors have no interests to declare.
Figures




Similar articles
-
Designing a hydrophobic barrier within biomimetic nanopores.ACS Nano. 2014 Nov 25;8(11):11268-79. doi: 10.1021/nn503930p. Epub 2014 Oct 20. ACS Nano. 2014. PMID: 25317664
-
Molecular simulation studies of hydrophobic gating in nanopores and ion channels.Biochem Soc Trans. 2015 Apr;43(2):146-50. doi: 10.1042/BST20140256. Biochem Soc Trans. 2015. PMID: 25849908 Review.
-
Voltage Gating of a Biomimetic Nanopore: Electrowetting of a Hydrophobic Barrier.ACS Nano. 2017 Feb 28;11(2):1840-1847. doi: 10.1021/acsnano.6b07865. Epub 2017 Feb 6. ACS Nano. 2017. PMID: 28141923
-
Water Nanoconfined in a Hydrophobic Pore: Molecular Dynamics Simulations of Transmembrane Protein 175 and the Influence of Water Models.ACS Nano. 2021 Dec 28;15(12):19098-19108. doi: 10.1021/acsnano.1c06443. Epub 2021 Nov 16. ACS Nano. 2021. PMID: 34784172 Free PMC article.
-
Water in Nanopores and Biological Channels: A Molecular Simulation Perspective.Chem Rev. 2020 Sep 23;120(18):10298-10335. doi: 10.1021/acs.chemrev.9b00830. Epub 2020 Aug 25. Chem Rev. 2020. PMID: 32841020 Free PMC article. Review.
Cited by
-
H+ and Confined Water in Gating in Many Voltage-Gated Potassium Channels: Ion/Water/Counterion/Protein Networks and Protons Added to Gate the Channel.Int J Mol Sci. 2025 Jul 29;26(15):7325. doi: 10.3390/ijms26157325. Int J Mol Sci. 2025. PMID: 40806455 Free PMC article. Review.
-
The structural basis of divalent cation block in a tetrameric prokaryotic sodium channel.Nat Commun. 2023 Jul 15;14(1):4236. doi: 10.1038/s41467-023-39987-0. Nat Commun. 2023. PMID: 37454189 Free PMC article.
-
Electronic Polarizability Tunes the Function of the Human Bestrophin 1 Cl- Channel.bioRxiv [Preprint]. 2024 Aug 8:2023.11.14.567055. doi: 10.1101/2023.11.14.567055. bioRxiv. 2024. Update in: J Chem Theory Comput. 2025 Jan 28;21(2):933-942. doi: 10.1021/acs.jctc.4c01039. PMID: 38014257 Free PMC article. Updated. Preprint.
-
Influence of electronic polarization on the binding of anions to a chloride-pumping rhodopsin.Biophys J. 2023 Apr 18;122(8):1548-1556. doi: 10.1016/j.bpj.2023.03.026. Epub 2023 Mar 21. Biophys J. 2023. PMID: 36945777 Free PMC article.
-
Electronic Polarizability Tunes the Function of the Human Bestrophin 1 Cl- Channel.J Chem Theory Comput. 2025 Jan 28;21(2):933-942. doi: 10.1021/acs.jctc.4c01039. Epub 2025 Jan 3. J Chem Theory Comput. 2025. PMID: 39754290 Free PMC article.
References
-
- Hille B. 3rd ed. Sinauer Associates Inc; 2001. Ionic Channels of Excitable Membranes.
-
- Ashcroft F. Academic Press; San Diego: 2000. Ion Channels and Disease.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

