Novel mRNA therapy restores GALT protein and enzyme activity in a zebrafish model of classic galactosemia
- PMID: 35527402
- PMCID: PMC9541528
- DOI: 10.1002/jimd.12512
Novel mRNA therapy restores GALT protein and enzyme activity in a zebrafish model of classic galactosemia
Abstract
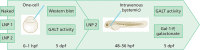
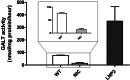
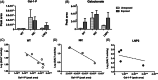
Messenger RNA (mRNA) has emerged as a novel therapeutic approach for inborn errors of metabolism. Classic galactosemia (CG) is an inborn error of galactose metabolism caused by a severe deficiency of galactose-1-phosphate:uridylyltransferase (GALT) activity leading to neonatal illness and chronic impairments affecting the brain and female gonads. In this proof of concept study, we used our zebrafish model for CG to evaluate the potential of human GALT mRNA (hGALT mRNA) packaged in two different lipid nanoparticles to restore GALT expression and activity at early stages of development. Both one cell-stage and intravenous single-dose injections resulted in hGALT protein expression and enzyme activity in the CG zebrafish (galt knockout) at 5 days post fertilization (dpf). Moreover, the levels of galactose-1-phosphate (Gal-1-P) and galactonate, metabolites that accumulate because of the deficiency, showed a decreasing trend. LNP-packaged mRNA was effectively translated and processed in the CG zebrafish without signs of toxicity. This study shows that mRNA therapy restores GALT protein and enzyme activity in the CG zebrafish model, and that the zebrafish is a suitable system to test this approach. Further studies are warranted to assess whether repeated injections safely mitigate the chronic impairments of this disease.
Keywords: GALT; classic galactosemia; lipid nanoparticles; mRNA; therapy; zebrafish.
© 2022 The Authors. Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Britt Delnoy, Minela Haskovic, Jo Vanoevelen, Laura K.M. Steinbusch, E. Naomi Vos, Kèvin Knoops, Luc JI Zimmermann, Marek Noga, Dirk J. Lefeber, Ana I Coelho, and M. Estela Rubio‐Gozalbo declare that they have no conflict of interest. Paolo G.V. Martini, a Moderna Therapeutics employee who provided the protocols and the mRNA (provided by Moderna Inc.) and who is listed as co‐author, was not involved in the conduction of the study.
Figures


Similar articles
-
Novel mRNA-Based Therapy Reduces Toxic Galactose Metabolites and Overcomes Galactose Sensitivity in a Mouse Model of Classic Galactosemia.Mol Ther. 2020 Jan 8;28(1):304-312. doi: 10.1016/j.ymthe.2019.09.018. Epub 2019 Sep 19. Mol Ther. 2020. PMID: 31604675 Free PMC article.
-
Restoring galactose metabolism without restoring GALT rescues both compromised survival in larvae and an adult climbing deficit in a GALT-null D. Melanogaster model of classic galactosemia.J Inherit Metab Dis. 2024 Sep;47(5):991-1000. doi: 10.1002/jimd.12774. Epub 2024 Jul 3. J Inherit Metab Dis. 2024. PMID: 38960603
-
AAV-mediated expression of galactose-1-phosphate uridyltransferase corrects defects of galactose metabolism in classic galactosemia patient fibroblasts.J Inherit Metab Dis. 2022 May;45(3):481-492. doi: 10.1002/jimd.12468. Epub 2022 Jan 23. J Inherit Metab Dis. 2022. PMID: 34918784
-
Hereditary galactosemia.Metabolism. 2018 Jun;83:188-196. doi: 10.1016/j.metabol.2018.01.025. Epub 2018 Jan 31. Metabolism. 2018. PMID: 29409891 Review.
-
Galactosemia: when is it a newborn screening emergency?Mol Genet Metab. 2012 May;106(1):7-11. doi: 10.1016/j.ymgme.2012.03.007. Epub 2012 Mar 21. Mol Genet Metab. 2012. PMID: 22483615 Review.
Cited by
-
Optical Coherence Tomography: Retinal Imaging Contributes to the Understanding of Brain Pathology in Classical Galactosemia.J Clin Med. 2023 Mar 3;12(5):2030. doi: 10.3390/jcm12052030. J Clin Med. 2023. PMID: 36902816 Free PMC article.
-
Reshaping the Treatment Landscape of a Galactose Metabolism Disorder.J Inherit Metab Dis. 2025 Mar;48(2):e70013. doi: 10.1002/jimd.70013. J Inherit Metab Dis. 2025. PMID: 39953772 Free PMC article. Review.
-
Evaluation of the efficacy of cystinosin supplementation through CTNS mRNA delivery in experimental models for cystinosis.Sci Rep. 2023 Nov 28;13(1):20961. doi: 10.1038/s41598-023-47085-w. Sci Rep. 2023. PMID: 38016974 Free PMC article.
-
Galactosemia: Biochemistry, Molecular Genetics, Newborn Screening, and Treatment.Biomolecules. 2022 Jul 11;12(7):968. doi: 10.3390/biom12070968. Biomolecules. 2022. PMID: 35883524 Free PMC article. Review.
-
Classical Hereditary galactosemia: findings in patients and animal models.Metab Brain Dis. 2024 Jan;39(1):239-248. doi: 10.1007/s11011-023-01281-9. Epub 2023 Sep 13. Metab Brain Dis. 2024. PMID: 37702899 Review.
References
-
- Kulkarni JA, Cullis PR, van der Meel R. Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucleic Acid Ther. 2018;28(3):146‐157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

