[Pan-cancer analysis of the expression pattern of long non-coding RNA MIR22HG]
- PMID: 35527483
- PMCID: PMC9085579
- DOI: 10.12122/j.issn.1673-4254.2022.04.03
[Pan-cancer analysis of the expression pattern of long non-coding RNA MIR22HG]
Abstract
Objective: To conduct a pan-cancer analysis of the expression of long non-coding RNA (lncRNA) MIR22HG and explore its association with clinical characteristics.
Methods: We analyzed the expression of MIR22HG in different tumors and its association with clinical staging, lymph node metastasis, tumor mutation burden (TMB) and microsatellite instability (MSI) using R package based on the Cancer Genome Atlas (TCGA) datasets. The relationship between MIR22HG expression and infiltrating immune cells was analyzed using TIMER algorithm. The association of MIR22HG gene alteration frequency with the clinical outcomes was examined using cBioPortal online software. Data form Genomics of Drug Sensitivity in Cancer (GDSC) were used to analyze the relationship between MIR22HG and the sensitivity of chemotherapy drugs. We specifically analyzed MIR22HG expression in hepatocellular carcinoma (HCC) and its correlation with sorafenib treatment using GEO database and verified the results in 12 pairs of HCC specimens. Kaplan-Meier analysis was performed to analyze the correlation of MIR22HG with the outcomes of sorafenib treatment. We also tested the effects of MIR22HG overexpression and knockdown on IC50 of sorafenib in HCC cells.
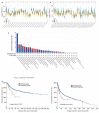
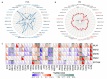
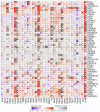
Results: MIR22HG was downregulated in most tumors (P < 0.05), where its deletion mutations were frequent, and associated with a poor prognosis (P < 0.05). In many tumors, MIR22HG expression level was correlated with clinical stage, lymph node metastasis, TMB, MSI, immune cell infiltration, immune checkpoint-related genes, and sensitivity to common chemotherapeutic drugs (P < 0.05). Among the 6 common infiltrating immune cells in cancers, neutrophil infiltration had the strongest correlation with MIR22HG expression level, especially in breast cancer, rectal cancer and kidney renal papillary cell carcinoma (P < 0.05). MIR22HG was downregulated in HCC in association with HCC progression (P < 0.05). In HCC patients, a low MIR22HG expression was associated with a favorable outcome after sorafenib treatment (HR=2.94, P=0.075) and was capable of predicting the response to sorafenib treatment (AUC=0.8095). Compared with the negative control, MIR22HG overexpression obviously reduced sorafenib sensitivity (with IC50 of 7.731 vs 15.61) while MIR22HG knockdown increased sorafenib sensitivity of HCC cells (with IC50 of 7.986 vs 5.085).
Conclusion: MIR22HG expression level is correlated with clinical stage, lymph node metastasis, TMB, MSI, immune cell infiltration, and chemosensitivity in most cancer, suggesting its potential as an immunotherapeutic target and also a prognostic biomarker for tumors.
目的: 泛癌分析长链非编码RNA(lncRNA)MIR22HG的表达及其与临床特征的关系。
方法: R语言分析癌症基因组图谱(TCGA)中MIR22HG在不同肿瘤组织中的表达及其与临床分期、淋巴结转移、肿瘤突变负荷(TMB)及微卫星不稳定(MSI)的关系;应用TIMER比较MIR22HG表达与免疫浸润的关系;使用cBioPortal分析MIR22HG的突变频率及其与预后的关系;使用GDSC在线数据库分析MIR22HG与化疗药物敏感性的关系。利用GEO数据库肝癌数据集和12对肝癌标本验证MIR22HG在肝癌中的表达及其与索拉非尼治疗反应的关系;分析MIR22HG表达与索拉非尼治疗预后的关系;在肝癌细胞株HCC-LM3中过表达MIR22HG(NC组,MIR22HG过表达组),在肝癌细胞株MHCC-97H中敲除MIR22HG(NC组,sh-MIR22HG组),采用CCK-8检测肝癌细胞中MIR22HG对索拉非尼IC50的影响。
结果: MIR22HG在大多数肿瘤中低表达(P<0.05),且多数肿瘤中MIR22HG基因存在缺失突变,其突变与肿瘤不良预后相关(P<0.05)。MIR22HG的表达水平与多种肿瘤的临床分期及淋巴结转移相关(P<0.05)。在多种肿瘤中MIR22HG的表达水平与TBM、MSI、免疫评分、免疫检查点相关基因表达及化疗药的药物敏感性显著相关(P<0.05)。6种常见免疫细胞中,中性粒细胞浸润水平与MIR22HG的表达水平相关性最强,尤其在乳腺癌、直肠癌及肾乳头状细胞癌中明显。多个数据集的分析结果验证MIR22HG在肝癌中低表达,且其低表达与肝癌进展相关(P<0.05)。MIR22HG低表达的患者经索拉非尼治疗后预后较好(HR=2.94, P=0.075),其低表达能有效预测肝癌患者索拉非尼治疗效果(AUC=0.8095)。过表达MIR22HG能降低肝癌细胞对索拉非尼的敏感性(IC50 NC vs IC50 MIR22HG=7.731 vs 15.61);而敲除MIR22HG的表达则能增加肝癌细胞对索拉非尼的敏感性(IC50 NC vs IC50sh-MIR22HG=7.986 vs 5.085)。
结论: MIR22HG的表达与多种肿瘤的分期、有否淋巴结转移、肿瘤突变负荷、微卫星不稳定、免疫细胞浸润及化疗药物敏感性相关。
Keywords: MIR22HG; hepatocellular carcinoma; long non-coding RNA; pan-cancer analysis; sorafenib.
Figures





Similar articles
-
Pan-Cancer Analysis of PARP1 Alterations as Biomarkers in the Prediction of Immunotherapeutic Effects and the Association of Its Expression Levels and Immunotherapy Signatures.Front Immunol. 2021 Aug 31;12:721030. doi: 10.3389/fimmu.2021.721030. eCollection 2021. Front Immunol. 2021. PMID: 34531868 Free PMC article.
-
Identification and Functional Characterization of Long Non-coding RNA MIR22HG as a Tumor Suppressor for Hepatocellular Carcinoma.Theranostics. 2018 Jun 13;8(14):3751-3765. doi: 10.7150/thno.22493. eCollection 2018. Theranostics. 2018. PMID: 30083257 Free PMC article.
-
LncRNA WAC-AS1 expression in human tumors correlates with immune infiltration and affects prognosis.Hereditas. 2023 May 30;160(1):26. doi: 10.1186/s41065-023-00290-z. Hereditas. 2023. PMID: 37248547 Free PMC article.
-
Upregulation of lncRNA NIFK-AS1 in hepatocellular carcinoma by m6A methylation promotes disease progression and sorafenib resistance.Hum Cell. 2021 Nov;34(6):1800-1811. doi: 10.1007/s13577-021-00587-z. Epub 2021 Aug 10. Hum Cell. 2021. PMID: 34374933 Review.
-
A Pan-Cancer Analysis Reveals the Prognostic and Immunotherapeutic Value of Stanniocalcin-2 (STC2).Front Genet. 2022 Jul 22;13:927046. doi: 10.3389/fgene.2022.927046. eCollection 2022. Front Genet. 2022. PMID: 35937984 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
