Parkinson's Disease Drug Therapies in the Clinical Trial Pipeline: 2022 Update
- PMID: 35527571
- PMCID: PMC9198738
- DOI: 10.3233/JPD-229002
Parkinson's Disease Drug Therapies in the Clinical Trial Pipeline: 2022 Update
Abstract
Background: As the international community dealt with the ongoing COVID-19 pandemic, important progress continued to be made in the development of new drug-based therapies for the neurodegenerative condition of Parkinson's disease (PD) in 2021. This progress included both "symptomatic treatments" (ST - improves/reduces symptoms of the condition) and "disease modifying treatments" (DMT - attempts to delay/slow progression by addressing the underlying biology of PD), which can be categorised further based on their mechanisms of action and class of drug.
Objective: This report continues previous efforts to provide an overview of the pharmacological therapies - both ST and DMT - in clinical trials for PD during 2021- 2022, with the aim of creating greater awareness and involvement in the clinical trial process. We also hope to stimulate collaboration amongst all stakeholders, including industry, academia, advocacy organizations, and most importantly patient community.
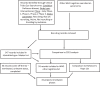
Methods: We conducted a review of clinical trials of drug therapies for PD using trial data obtained from the ClinicalTrials.gov and World Health Organisation (WHO) registries, and performed a breakdown analysis of studies that were active as of January 31st 2022. We also assessed active drug development projects that had completed one clinical phase but were yet to start the next.
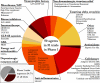
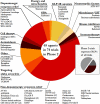
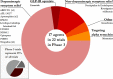
Results: There was a total of 147 clinical trials registered on the ClinicalTrials.gov website as active during the period of analysis. Of these trials, 91 (62%)were investigating STs, while 56 (38%)focused on DMTs. Approximately 1/3 of the studies (34.7%; 51 trials) were in Phase 1, while over half of the trials were in Phase 2 (50.3%; 74 trials). Only 15% (22 trials) of the studies were in Phase 3, of which only 3 trials were evaluating DMTs. Novel therapeutics (42%)were the most common type of agents being tested across all phases of testing, followed by repurposed agents (34%)and reformulations (20%).
Conclusion: Despite significant global health constraints, the development of new drug-based therapies for PD continued in 2021. Hopefully with a shift towards a post-pandemic world in which COVID-19 is better managed, we will see an increase in the number of clinical trials focused on drug development for PD. The need for more Phase 3 studies for DMTs remains acute.
Keywords: Clinical trials; Parkinson’s; disease modification; gene therapy; immunotherapy; inflammation; neuroprotection; studies.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- WHO, recommends groundbreaking malaria vaccine for children at risk, http://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-ma...
-
- Esrick EB, Lehmann LE, Biffi A, Achebe M, Brendel C, Ciuculescu MF, Daley H, MacKinnon B, Morris E, Federico A, Abriss D, Boardman K, Khelladi R, Shaw K, Negre H, Negre O, Nikiforow S, Ritz J, Pai S-Y, London WB, Dansereau C, Heeney MM, Armant M, Manis JP, Williams DA (2021) Post-Transcriptional Genetic Silencing of BCL11A to Treat Sickle Cell Disease. N Engl J Med 384(3), 205–215. - PMC - PubMed
-
- Andrieux-Meyer I, Tan SS, Thanprasertsuk S, Salvadori N, Menétrey C, Simon F, Cressey TR, Said HRHM, Hassan MRA, Omar H, Tee HP, Chan WK, Kumar S, Thongsawat S, Thetket K, Avihingsanon A, Khemnark S, Yerly S, Ngo-Giang-Huong N, Siva S, Swanson A, Goyal V, Bompart F, Pécoul B, Murad S (2021) Efficacy and safety ofravidasvir plus sofosbuvir in patients with chronic hepatitis Cinfection without cirrhosis or with compensated cirrhosis(STORM-C-1): Interim analysis of a two-stage, open-label,multicentre, single arm, phase 2/3 trial. Lancet GastroenterolHepatol 6(6), 448–458. - PMC - PubMed
-
- Grantees for Circuitry and Brain-Body Interactions, http://parkinsonsroadmap.org/research-network/circuitry/
-
- Edmond J. Safra Foundation awards £1.375 million to rapidly accelerate Parkinson’s drug search http://www.ucl.ac.uk/campaign/news/2021/jun/edmond-j-safra-foundation-aw...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

