Control of Behavioral Arousal and Defense by a Glutamatergic Midbrain-Amygdala Pathway in Mice
- PMID: 35527820
- PMCID: PMC9070111
- DOI: 10.3389/fnins.2022.850193
Control of Behavioral Arousal and Defense by a Glutamatergic Midbrain-Amygdala Pathway in Mice
Abstract
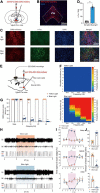
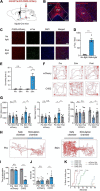
In response to external threatening signals, animals evolve a series of defensive behaviors that depend on heightened arousal. It is believed that arousal and defensive behaviors are coordinately regulated by specific neurocircuits in the central nervous system. The ventral tegmental area (VTA) is a key structure located in the ventral midbrain of mice. The activity of VTA glutamatergic neurons has recently been shown to be closely related to sleep-wake behavior. However, the specific role of VTA glutamatergic neurons in sleep-wake regulation, associated physiological functions, and underlying neural circuits remain unclear. In the current study, using an optogenetic approach and synchronous polysomnographic recording, we demonstrated that selective activation of VTA glutamatergic neurons induced immediate transition from sleep to wakefulness and obviously increased the amount of wakefulness in mice. Furthermore, optogenetic activation of VTA glutamatergic neurons induced multiple defensive behaviors, including burrowing, fleeing, avoidance and hiding. Finally, viral-mediated anterograde activation revealed that projections from the VTA to the central nucleus of the amygdala (CeA) mediated the wake- and defense-promoting effects of VTA glutamatergic neurons. Collectively, our results illustrate that the glutamatergic VTA is a key neural substrate regulating wakefulness and defensive behaviors that controls these behaviors through its projection into the CeA. We further discuss the possibility that the glutamatergic VTA-CeA pathway may be involved in psychiatric diseases featuring with excessive defense.
Keywords: autism spectrum disorders; central nucleus of the amygdala; defensive behavior; sleep-wake behavior; ventral tegmental area.
Copyright © 2022 Chen, Yao, Hu, Chen, Liu, Wang, Zeng, Zhuang, Zeng, Li, Yang, Huang, Huang, Lai, Hu, Cai, Chen and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
A glutamatergic basal forebrain to midbrain circuit mediates wakefulness and defensive behavior.Neuropharmacology. 2022 May 1;208:108979. doi: 10.1016/j.neuropharm.2022.108979. Epub 2022 Feb 4. Neuropharmacology. 2022. PMID: 35131297
-
Ventral Tegmental Area Glutamatergic Neurons Facilitated Emergence From Isoflurane Anesthesia Involves Excitation of Lateral Septum GABA-ergic Neurons in Mice.Anesth Analg. 2024 Aug 1;139(2):397-410. doi: 10.1213/ANE.0000000000006739. Epub 2023 Nov 27. Anesth Analg. 2024. PMID: 38048607
-
VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors.Nat Neurosci. 2016 Oct;19(10):1356-66. doi: 10.1038/nn.4377. Epub 2016 Sep 5. Nat Neurosci. 2016. PMID: 27595385 Free PMC article.
-
Role of the ventral tegmental area in general anesthesia.Eur J Pharmacol. 2025 Jan 5;986:177145. doi: 10.1016/j.ejphar.2024.177145. Epub 2024 Nov 19. Eur J Pharmacol. 2025. PMID: 39566814 Review.
-
[Neurochemical mechanisms of sleep regulation].Glas Srp Akad Nauka Med. 2009;(50):97-109. Glas Srp Akad Nauka Med. 2009. PMID: 20666118 Review. Serbian.
Cited by
-
GABAergic Neurons in the Central Amygdala Promote Emergence from Isoflurane Anesthesia in Mice.Anesthesiology. 2025 Feb 1;142(2):278-297. doi: 10.1097/ALN.0000000000005279. Epub 2024 Oct 28. Anesthesiology. 2025. PMID: 39466630 Free PMC article.
-
Central amygdala somatostatin neurons modulate stress-induced sleep-onset insomnia.Commun Biol. 2025 Mar 6;8(1):381. doi: 10.1038/s42003-025-07679-8. Commun Biol. 2025. PMID: 40050691 Free PMC article.
-
Defensive responses: behaviour, the brain and the body.Nat Rev Neurosci. 2023 Nov;24(11):655-671. doi: 10.1038/s41583-023-00736-3. Epub 2023 Sep 20. Nat Rev Neurosci. 2023. PMID: 37730910 Review.
-
The Formation and Function of the VTA Dopamine System.Int J Mol Sci. 2024 Mar 30;25(7):3875. doi: 10.3390/ijms25073875. Int J Mol Sci. 2024. PMID: 38612683 Free PMC article. Review.
-
Parabrachial nucleus Vglut2 expressing neurons projection to the extended amygdala involved in the regulation of wakefulness during sevoflurane anesthesia in mice.CNS Neurosci Ther. 2024 Aug;30(8):e70001. doi: 10.1111/cns.70001. CNS Neurosci Ther. 2024. PMID: 39154359 Free PMC article.
References
LinkOut - more resources
Full Text Sources

