Facile synthesis of few-layer MoS2 in MgAl-LDH layers for enhanced visible-light photocatalytic activity
- PMID: 35527890
- PMCID: PMC9069589
- DOI: 10.1039/c9ra03858b
Facile synthesis of few-layer MoS2 in MgAl-LDH layers for enhanced visible-light photocatalytic activity
Abstract
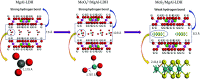
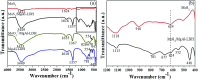
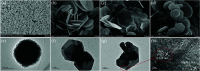
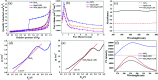
A new photocatalyst, few-layer MoS2 grown in MgAl-LDH interlayers (MoS2/MgAl-LDH), was prepared by a facile two-step hydrothermal synthesis. The structural and photocatalytic properties of the obtained material were characterized by several techniques including powder X-ray diffraction (XRD), Raman spectroscopy, field emission scanning electron microscopy (FESEM), high-resolution transmission electron microscopy (HRTEM), Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), photoluminescence spectroscopy (PL) and UV-vis absorption spectroscopy. The MoS2/MgAl-LDH composite showed excellent photocatalytic performance for methyl orange (MO) degradation at low concentrations (50 mg L-1 and 100 mg L-1). Furthermore, even for a MO solution concentration as high as 200 mg L-1, this composite also presented high degradation efficiency (>84%) and mineralization efficiency (>73%) at 120 min. The results show that the MoS2/MgAl-LDH composite has great potential for application in wastewater treatment.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Iqbal K. Iqbal A. Kirillov A. M. Wang B. Liu W. Tang Y. J. Mater. Chem. A. 2017;5:6716–6724. doi: 10.1039/C6TA10880F. - DOI
-
- Valentea J. S. Tzompantzib F. Princec J. Appl. Catal., B. 2011;102:276–285. doi: 10.1016/j.apcatb.2010.12.009. - DOI
-
- Liu X. Zhao X. Zhu Y. Zhang F. Appl. Catal., B. 2013;140:241–248. doi: 10.1016/j.apcatb.2013.04.008. - DOI
-
- Legrini O. Oliveros E. Braun A. M. Chem. Rev. 1993;93:671–698. doi: 10.1021/cr00018a003. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

