Synthesis of a high-surface area V2O5/TiO2-SiO2 catalyst and its application in the visible light photocatalytic degradation of methylene blue
- PMID: 35527892
- PMCID: PMC9069717
- DOI: 10.1039/c9ra03866c
Synthesis of a high-surface area V2O5/TiO2-SiO2 catalyst and its application in the visible light photocatalytic degradation of methylene blue
Abstract
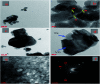
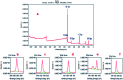
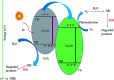
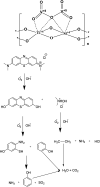
In the present study, we synthesized several high-surface area V2O5/TiO2-SiO2 catalysts (vanado titanium silicate, VTS). The synthesized materials were characterized by PXRD, FE-SEM/EDAX, TEM, FTIR, UV-Vis, XPS, fluorescence and photocatalytic activity studies. The small-angle powder X-ray diffraction pattern shows that the 110 and 200 planes are merged to become a single broad peak. Field-emission scanning electron microscopy shows that the titanosilicate is spherical in shape and V2O5 has a hexagonal rod-shaped morphology. The presence of various metal ions, such as V, Ti, Si and O, was observed by energy dispersive X-ray analysis and X-ray photoelectron spectroscopy. The transmission electron microscopy image shows clear hexagonal mesoporous fringes with V2O5 distribution. The BET surface area analysis shows that the VTS catalysts have higher surface areas than pure V2O5. Fourier transform infrared spectroscopic analysis shows the presence of Ti4+ ions connected to the silanol groups. The bandwidths of pure titanosilicate, V2O5 and their composites were calculated from their diffuse reflectance ultraviolet-visible spectra. The bandwidth was tuned by heterojunctions in the studied catalysts. The photoluminescence spectra of the VTS catalysts show a distinct behaviour as compared to those of the pure components. The photocatalytic activity of methylene blue degradation was determined with pure constituents and catalysts. VTS-1 (TS and VO weight ratio 2 : 1) shows higher conversion than other catalysts, pure titanosilicate and V2O5. This is probably due to the heterojunctions and higher surface area in VTS-1. Kinetic studies reveal that direct sunlight shows higher activity than pure visible light. A plausible physical and chemical mechanism for the photocatalytic activity is proposed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Mills A. Le Hunte S. J. Photochem. Photobiol., A. 1997;108:1–35. doi: 10.1016/S1010-6030(97)00118-4. - DOI
-
- Fujishima A. Zhang X. Tryk D. Int. J. Hydrogen Energy. 2007;32:2664–2672. doi: 10.1016/j.ijhydene.2006.09.009. - DOI
-
- Bahnemann D. Sol. Energy. 2004;77:445–459. doi: 10.1016/j.solener.2004.03.031. - DOI
-
- Hoffmann M. R. Martin S. T. Choi W. Bahnemann D. W. Chem. Rev. 1995;95:69–96. doi: 10.1021/cr00033a004. - DOI
LinkOut - more resources
Full Text Sources

