Enantioselective bromination of axially chiral cyanoarenes in the presence of bifunctional organocatalysts
- PMID: 35527922
- PMCID: PMC9072646
- DOI: 10.1039/c9ra05532k
Enantioselective bromination of axially chiral cyanoarenes in the presence of bifunctional organocatalysts
Abstract
Enantioselective bromination of axially chiral cyanoarenes bearing high intrinsic rotational barriers via dynamic kinetic resolution using bifunctional organocatalysts is reported. Sequential addition of a brominating reagent in several portions at an optimized temperature was effective in accomplishing high enantioselectivities.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

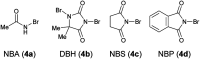
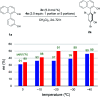




References
-
- Bringmann G. Menche D. Acc. Chem. Res. 2001;34:615. doi: 10.1021/ar000106z. - DOI - PubMed
- Kozlowski M. C. Morgan B. J. Linton E. C. Chem. Soc. Rev. 2009;38:3193. doi: 10.1039/B821092F. - DOI - PMC - PubMed
- Bringmann G. Gulder T. Gulder T. A. M. Breuning M. Chem. Rev. 2011;111:563. doi: 10.1021/cr100155e. - DOI - PubMed
- Zask A. Murphy J. Ellestad G. A. Chirality. 2013;25:265. doi: 10.1002/chir.22145. - DOI - PubMed
- Smyth J. E. Butler N. M. Keller P. A. Nat. Prod. Rep. 2015;32:1562. doi: 10.1039/C4NP00121D. - DOI - PubMed
- Covington C. L. Junior F. M. S. Silva J. H. S. Kuster R. M. de Amorim M. B. Polavarapu P. L. J. Nat. Prod. 2016;79:2530. doi: 10.1021/acs.jnatprod.6b00395. - DOI - PubMed
-
- McCarthy M. Guiry P. J. Tetrahedron. 2001;57:3809. doi: 10.1016/S0040-4020(01)00087-4. - DOI
- Chen Y. Yekta S. Yudin A. K. Chem. Rev. 2003;103:3155. doi: 10.1021/cr020025b. - DOI - PubMed
- Brunel J. M. Chem. Rev. 2005;105:857. doi: 10.1021/cr040079g. - DOI - PubMed
- Yoon T. P. Jacobsen E. N. Science. 2003;299:1691. doi: 10.1126/science.1083622. - DOI - PubMed
-
- Li Q. Green L. Venkataraman N. Shiyanovskaya I. Khan A. Urbas A. Doane J. W. J. Am. Chem. Soc. 2007;129:12908. doi: 10.1021/ja0747573. - DOI - PubMed
- Hayasaka H. Miyashita T. Nakayama M. Kuwada K. Akagi K. J. Am. Chem. Soc. 2012;134:3758. doi: 10.1021/ja2088053. - DOI - PubMed
- Wu Y.-L. Ferroni F. Pieraccini S. Schweizer W. B. Frank B. B. Spada G. P. Diederich F. Org. Biomol. Chem. 2012;10:8016. doi: 10.1039/C2OB25983D. - DOI - PubMed
- Pu L. Acc. Chem. Res. 2012;45:150. doi: 10.1021/ar200048d. - DOI - PubMed
- Wei G. Zhang S. Dai C. Quan Y. Cheng Y. Zhu C. Chem.–Eur. J. 2013;19:16066. doi: 10.1002/chem.201302726. - DOI - PubMed
-
-
For selected recent reviews on enantioselective syntheses of axially chiral compounds, see ref. 1 and the following:
- Cozzi P. G. Emer E. Gualandi A. Angew. Chem., Int. Ed. 2011;50:3847. doi: 10.1002/anie.201008031. - DOI - PubMed
- Quinonero O. Bressy C. Bugaut X. Angew. Chem., Int. Ed. 2014;53:10861. doi: 10.1002/anie.201406263. - DOI - PubMed
- Wencel-Delord J. Panossian A. Leroux F. R. Colobert F. Chem. Soc. Rev. 2015;44:3418. doi: 10.1039/C5CS00012B. - DOI - PubMed
- Ma G. Sibi M. P. Chem.–Eur. J. 2015;21:11644. doi: 10.1002/chem.201500869. - DOI - PubMed
- Bencivenni G. Synlett. 2015;26:1915. doi: 10.1055/s-0034-1378712. - DOI
- Kumarasamy E. Raghunathan R. Sibi M. P. Sivaguru J. Chem. Rev. 2015;115:11239. doi: 10.1021/acs.chemrev.5b00136. - DOI - PubMed
- Shirakawa S. Liu S. Kaneko S. Chem.–Asian J. 2016;11:330. doi: 10.1002/asia.201500951. - DOI - PubMed
- Renzi P. Org. Biomol. Chem. 2017;15:4506. doi: 10.1039/C7OB00908A. - DOI - PubMed
- Zilate B. Castrogiovanni A. Sparr C. ACS Catal. 2018;8:2981. doi: 10.1021/acscatal.7b04337. - DOI
- Baudoin O. Eur. J. Org. Chem. 2005:4223. doi: 10.1002/ejoc.200500394. - DOI
- Bringmann G. Mortimer A. J. P. Keller P. A. Gresser M. J. Garner J. Breuning M. Angew. Chem., Int. Ed. 2005;44:5384. doi: 10.1002/anie.200462661. - DOI - PubMed
- Zhang D. Wang Q. Coord. Chem. Rev. 2015;286:1. doi: 10.1016/j.ccr.2014.11.011. - DOI
- Cherney A. H. Kadunce N. T. Reisman S. E. Chem. Rev. 2015;115:9587. doi: 10.1021/acs.chemrev.5b00162. - DOI - PMC - PubMed
- Loxq P. Manoury E. Poli R. Deydier E. Labande A. Coord. Chem. Rev. 2016;308:131. doi: 10.1016/j.ccr.2015.07.006. - DOI
- Tanaka K. Chem.–Asian J. 2009;4:508. doi: 10.1002/asia.200800378. - DOI - PubMed
-
-
- Gustafson J. L. Lim D. Miller S. J. Science. 2010;328:1251. doi: 10.1126/science.1188403. - DOI - PMC - PubMed
- Pathak T. P. Miller S. J. J. Am. Chem. Soc. 2012;134:6120. doi: 10.1021/ja301566t. - DOI - PMC - PubMed
- Barrett K. T. Miller S. J. J. Am. Chem. Soc. 2013;135:2963. doi: 10.1021/ja400082x. - DOI - PMC - PubMed
- Barrett K. T. Metrano A. J. Rablen P. R. Miller S. J. Nature. 2014;509:71. doi: 10.1038/nature13189. - DOI - PMC - PubMed
- Diener M. E. Metrano A. J. Kusano S. Miller S. J. J. Am. Chem. Soc. 2015;137:12369. doi: 10.1021/jacs.5b07726. - DOI - PMC - PubMed
- Miyaji R. Asano K. Matsubara S. J. Am. Chem. Soc. 2015;137:6766. doi: 10.1021/jacs.5b04151. - DOI - PubMed
- Miyaji R. Asano K. Matsubara S. Chem.–Eur. J. 2017;23:9996. doi: 10.1002/chem.201701707. - DOI - PubMed
- Miyaji R. Wada Y. Matsumoto A. Asano K. Matsubara S. Beilstein J. Org. Chem. 2017;13:1518. doi: 10.3762/bjoc.13.151. - DOI - PMC - PubMed

