Neuroendocrine Regulation of Plasma Cortisol Levels During Smoltification and Seawater Acclimation of Atlantic Salmon
- PMID: 35528002
- PMCID: PMC9069684
- DOI: 10.3389/fendo.2022.859817
Neuroendocrine Regulation of Plasma Cortisol Levels During Smoltification and Seawater Acclimation of Atlantic Salmon
Abstract
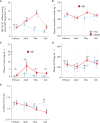
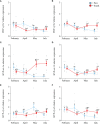
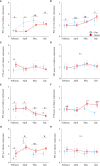
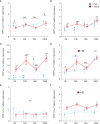
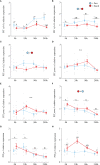
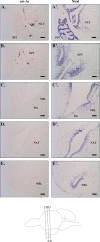
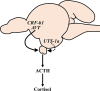
Diadromous fishes undergo dramatic changes in osmoregulatory capacity in preparation for migration between freshwater and seawater. One of the primary hormones involved in coordinating these changes is the glucocorticoid hormone, cortisol. In Atlantic salmon (Salmo salar), cortisol levels increase during the spring smoltification period prior to seawater migration; however, the neuroendocrine factors responsible for regulating the hypothalamic-pituitary-interrenal (HPI) axis and plasma cortisol levels during smoltification remain unclear. Therefore, we evaluated seasonal changes in circulating levels of cortisol and its primary secretagogue-adrenocorticotropic hormone (ACTH)-as well as transcript abundance of the major regulators of HPI axis activity in the preoptic area, hypothalamus, and pituitary between migratory smolts and pre-migratory parr. Smolts exhibited higher plasma cortisol levels compared to parr across all timepoints but circulating ACTH levels were only elevated in May. Transcript abundance of preoptic area corticotropin-releasing factor b1 and arginine vasotocin were ~2-fold higher in smolts compared to parr in February through May. Smolts also had ~7-fold greater hypothalamic transcript abundance of urotensin 1 (uts-1a) compared to parr in May through July. When transferred to seawater during peak smolting in May smolts rapidly upregulated hypothalamic uts-1a transcript levels within 24 h, while parr only transiently upregulated uts-1a 96 h post-transfer. In situ hybridization revealed that uts-1a is highly abundant in the lateral tuberal nucleus (NLT) of the hypothalamus, consistent with a role in regulating the HPI axis. Overall, our results highlight the complex, multifactorial regulation of cortisol and provide novel insight into the neuroendocrine mechanisms controlling osmoregulation in teleosts.
Keywords: Salmo salar; adrenocorticotropic hormone; arginine vasotocin; corticotropin-releasing factor; cortisol; urotensin 1.
Copyright © 2022 Culbert, Regish, Hall, McCormick and Bernier.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Zydlewski J, Wilkie MP. “Freshwater to Seawater Transitions in Migratory Fishes”. In: Euryhaline Fishes. Oxford, UK: Academic Press; (2012). p. 253–326. doi: 10.1016/B978-0-12-396951-4.00006-2 - DOI
-
- Shrimpton JM. “Seawater to Freshwater Transitions in Diadromous Fishes,”. In: Euryhaline Fishes. Oxford, UK: Academic Press; (2012). p. 327–93. doi: 10.1016/B978-0-12-396951-4.00007-4 - DOI
-
- Takei Y, Loretz CA. “The Gastrointestinal Tract as an Endocrine/Neuroendocrine/Paracrine Organ: Organization, Chemical Messengers and Physiological Targets”. In: The Multifunctional Gut of Fish. London, UK: Academic Press; (2010). p. 261–317. doi: 10.1016/S1546-5098(10)03007-4 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

