Live-cell fluorescence spectral imaging as a data science challenge
- PMID: 35528031
- PMCID: PMC9043069
- DOI: 10.1007/s12551-022-00941-x
Live-cell fluorescence spectral imaging as a data science challenge
Abstract
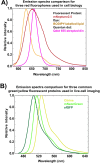
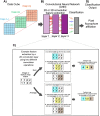
Live-cell fluorescence spectral imaging is an evolving modality of microscopy that uses specific properties of fluorophores, such as excitation or emission spectra, to detect multiple molecules and structures in intact cells. The main challenge of analyzing live-cell fluorescence spectral imaging data is the precise quantification of fluorescent molecules despite the weak signals and high noise found when imaging living cells under non-phototoxic conditions. Beyond the optimization of fluorophores and microscopy setups, quantifying multiple fluorophores requires algorithms that separate or unmix the contributions of the numerous fluorescent signals recorded at the single pixel level. This review aims to provide both the experimental scientist and the data analyst with a straightforward description of the evolution of spectral unmixing algorithms for fluorescence live-cell imaging. We show how the initial systems of linear equations used to determine the concentration of fluorophores in a pixel progressively evolved into matrix factorization, clustering, and deep learning approaches. We outline potential future trends on combining fluorescence spectral imaging with label-free detection methods, fluorescence lifetime imaging, and deep learning image analysis.
Keywords: Cell biology; Data science; Fluorescence; Live-cell imaging; Spectral imaging.
© International Union for Pure and Applied Biophysics (IUPAB) and Springer-Verlag GmbH Germany, part of Springer Nature 2022.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures




Similar articles
-
Robust blind spectral unmixing for fluorescence microscopy using unsupervised learning.PLoS One. 2019 Dec 2;14(12):e0225410. doi: 10.1371/journal.pone.0225410. eCollection 2019. PLoS One. 2019. PMID: 31790435 Free PMC article.
-
Multiplexed imaging in live cells using pulsed interleaved excitation spectral FLIM.Opt Express. 2024 Jan 29;32(3):3290-3307. doi: 10.1364/OE.505667. Opt Express. 2024. PMID: 38297554 Free PMC article.
-
Semi-blind sparse affine spectral unmixing of autofluorescence-contaminated micrographs.Bioinformatics. 2020 Feb 1;36(3):910-917. doi: 10.1093/bioinformatics/btz674. Bioinformatics. 2020. PMID: 31504202 Free PMC article.
-
Applications of combined spectral lifetime microscopy for biology.Biotechniques. 2006 Sep;41(3):249, 251, 253 passim. doi: 10.2144/000112251. Biotechniques. 2006. PMID: 16989084 Review.
-
Spectral imaging and its applications in live cell microscopy.FEBS Lett. 2003 Jul 3;546(1):87-92. doi: 10.1016/s0014-5793(03)00521-0. FEBS Lett. 2003. PMID: 12829241 Review.
Cited by
-
Environmental challenge trials induce a biofluorescent response in the green sea urchin Strongylocentrotus droebachiensis.Sci Rep. 2024 Nov 4;14(1):26671. doi: 10.1038/s41598-024-77648-4. Sci Rep. 2024. PMID: 39496746 Free PMC article.
-
Super-multiplexing excitation spectral microscopy with multiple fluorescence bands.Biomed Opt Express. 2022 Oct 27;13(11):6048-6060. doi: 10.1364/BOE.473241. eCollection 2022 Nov 1. Biomed Opt Express. 2022. PMID: 36733753 Free PMC article.
-
Probing organoid metabolism using fluorescence lifetime imaging microscopy (FLIM): The next frontier of drug discovery and disease understanding.Adv Drug Deliv Rev. 2023 Oct;201:115081. doi: 10.1016/j.addr.2023.115081. Epub 2023 Aug 28. Adv Drug Deliv Rev. 2023. PMID: 37647987 Free PMC article. Review.
-
Closing the multichannel gap through computational reconstruction of interaction in super-resolution microscopy.Patterns (N Y). 2025 Mar 27;6(5):101181. doi: 10.1016/j.patter.2025.101181. eCollection 2025 May 9. Patterns (N Y). 2025. PMID: 40486966 Free PMC article. Review.
-
A Bright, Photostable, and Far-Red Dye That Enables Multicolor, Time-Lapse, and Super-Resolution Imaging of Acidic Organelles.ACS Cent Sci. 2023 Dec 14;10(1):19-27. doi: 10.1021/acscentsci.3c01173. eCollection 2024 Jan 24. ACS Cent Sci. 2023. PMID: 38292604 Free PMC article.
References
-
- Abdeladim L, Matho KS, Clavreul S, Mahou P, Sintes JM, Solinas X, Arganda-Carreras I, Turney SG, Lichtman JW, Chessel A, Bemelmans AP, Loulier K, Supatto W, Livet J, Beaurepaire E. Multicolor multiscale brain imaging with chromatic multiphoton serial microscopy. Nat Commun. 2019;10:1662. doi: 10.1038/s41467-019-09552-9. - DOI - PMC - PubMed
-
- Aggarwal HK, Majumdar A. Hyperspectral unmixing in the presence of mixed noise using joint-sparsity and total variation. IEEE Journal of Selected Topics in Applied Earth Observations and Remote Sensing. 2016;9:4257–4266. doi: 10.1109/JSTARS.2016.2521898. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

