Structural origin of cooperativity in human hemoglobin: a view from different roles of α and β subunits in the α2β2 tetramer
- PMID: 35528033
- PMCID: PMC9043147
- DOI: 10.1007/s12551-022-00945-7
Structural origin of cooperativity in human hemoglobin: a view from different roles of α and β subunits in the α2β2 tetramer
Abstract
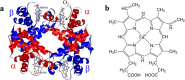
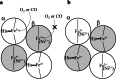
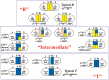
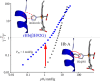
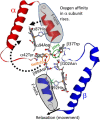
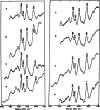
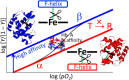
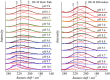
This mini-review, mainly based on our resonance Raman studies on the structural origin of cooperative O2 binding in human adult hemoglobin (HbA), aims to answering why HbA is a tetramer consisting of two α and two β subunits. Here, we focus on the Fe-His bond, the sole coordination bond connecting heme to a globin. The Fe-His stretching frequencies reflect the O2 affinity and also the magnitude of strain imposed through globin by inter-subunit interactions, which is the origin of cooperativity. Cooperativity was first explained by Monod, Wyman, and Changeux, referred to as the MWC theory, but later explained by the two tertiary states (TTS) theory. Here, we related the higher-order structures of globin observed mainly by vibrational spectroscopy to the MWC theory. It became clear from the recent spectroscopic studies, X-ray crystallographic analysis, and mutagenesis experiments that the Fe-His bonds exhibit different roles between the α and β subunits. The absence of the Fe-His bond in the α subunit in some mutant and artificial Hbs inhibits T to R quaternary structural change upon O2 binding. However, its absence from the β subunit in mutant and artificial Hbs simply enhances the O2 affinity of the α subunit. Accordingly, the inter-subunit interactions between α and β subunits are nonsymmetric but substantial for HbA to perform cooperative O2 binding.
Keywords: Cooperativity; Hemoglobin; Iron-histidine bond; Quaternary structure; Resonance Raman; Subunits.
© International Union for Pure and Applied Biophysics (IUPAB) and Springer-Verlag GmbH Germany, part of Springer Nature 2022.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures

Similar articles
-
An Origin of Cooperative Oxygen Binding of Human Adult Hemoglobin: Different Roles of the α and β Subunits in the α2β2 Tetramer.PLoS One. 2015 Aug 5;10(8):e0135080. doi: 10.1371/journal.pone.0135080. eCollection 2015. PLoS One. 2015. PMID: 26244770 Free PMC article.
-
Heterogeneity between Two α Subunits of α2β2 Human Hemoglobin and O2 Binding Properties: Raman, 1H Nuclear Magnetic Resonance, and Terahertz Spectra.Biochemistry. 2017 Nov 21;56(46):6125-6136. doi: 10.1021/acs.biochem.7b00733. Epub 2017 Nov 7. Biochemistry. 2017. PMID: 29064674
-
Structural heterogeneity of the Fe(2+)-N epsilon (HisF8) bond in various hemoglobin and myoglobin derivatives probed by the Raman-active iron histidine stretching mode.Biophys J. 1993 Oct;65(4):1470-85. doi: 10.1016/S0006-3495(93)81216-5. Biophys J. 1993. PMID: 8274641 Free PMC article.
-
Evolution of allosteric models for hemoglobin.IUBMB Life. 2007 Aug-Sep;59(8-9):586-99. doi: 10.1080/15216540701272380. IUBMB Life. 2007. PMID: 17701554 Review.
-
How does hemoglobin generate such diverse functionality of physiological relevance?Biochim Biophys Acta. 2013 Sep;1834(9):1873-84. doi: 10.1016/j.bbapap.2013.04.026. Epub 2013 May 1. Biochim Biophys Acta. 2013. PMID: 23643742 Review.
Cited by
-
Orphan Nuclear Receptors TR2 and TR4 in Erythropoiesis: From Mechanisms to Therapies.Biomolecules. 2025 May 31;15(6):798. doi: 10.3390/biom15060798. Biomolecules. 2025. PMID: 40563438 Free PMC article. Review.
-
Biophysical Reviews: focusing on an issue.Biophys Rev. 2022 Apr 19;14(2):413-416. doi: 10.1007/s12551-022-00953-7. eCollection 2022 Apr. Biophys Rev. 2022. PMID: 35528037 Free PMC article.
-
Study of N-Acetylamino Saccharides with Synchrotron-Based Ultraviolet Resonance Raman Spectroscopy: In Combination with ATR Far-Ultraviolet Spectroscopy.J Phys Chem Lett. 2025 Feb 20;16(7):1769-1778. doi: 10.1021/acs.jpclett.4c03435. Epub 2025 Feb 12. J Phys Chem Lett. 2025. PMID: 39936528 Free PMC article.
-
Precise Engineering and Efficient Biosynthesis of Robust and High-Activity Human Haemoglobin for Artificial Oxygen Carriers.Microb Biotechnol. 2025 Mar;18(3):e70128. doi: 10.1111/1751-7915.70128. Microb Biotechnol. 2025. PMID: 40072822 Free PMC article.
-
Unusual one dimensional cascade effect in the thermal and photo-induced switching of azobenzene derivatives on a graphite surface.Chem Sci. 2025 Mar 5;16(15):6325-6335. doi: 10.1039/d4sc07570f. eCollection 2025 Apr 9. Chem Sci. 2025. PMID: 40083970 Free PMC article.
References
-
- Adair GS. The hemoglobin system. VI. The oxygen dissociation curve of hemoglobin. J Biol Chem. 1925;63:529–545. doi: 10.1016/S0021-9258(18)85018-9. - DOI
-
- Balakrishnan G, Case MA, Pevsner A, Zhao X, Tengroth C, McLendon GL, Spiro TG. Time-resolved absorption and UV resonance Raman spectra reveal stepwise formation of T quaternary contacts in the allosteric pathway of hemoglobin. J Mol Biol. 2004;340:843–856. doi: 10.1016/j.jmb.2004.05.012. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

