Structural origin of cooperativity in human hemoglobin: a view from different roles of α and β subunits in the α2β2 tetramer
- PMID: 35528033
- PMCID: PMC9043147
- DOI: 10.1007/s12551-022-00945-7
Structural origin of cooperativity in human hemoglobin: a view from different roles of α and β subunits in the α2β2 tetramer
Abstract
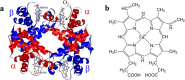
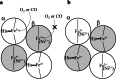
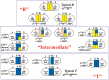
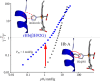
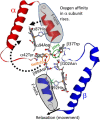
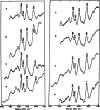
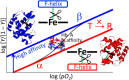
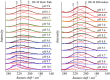
This mini-review, mainly based on our resonance Raman studies on the structural origin of cooperative O2 binding in human adult hemoglobin (HbA), aims to answering why HbA is a tetramer consisting of two α and two β subunits. Here, we focus on the Fe-His bond, the sole coordination bond connecting heme to a globin. The Fe-His stretching frequencies reflect the O2 affinity and also the magnitude of strain imposed through globin by inter-subunit interactions, which is the origin of cooperativity. Cooperativity was first explained by Monod, Wyman, and Changeux, referred to as the MWC theory, but later explained by the two tertiary states (TTS) theory. Here, we related the higher-order structures of globin observed mainly by vibrational spectroscopy to the MWC theory. It became clear from the recent spectroscopic studies, X-ray crystallographic analysis, and mutagenesis experiments that the Fe-His bonds exhibit different roles between the α and β subunits. The absence of the Fe-His bond in the α subunit in some mutant and artificial Hbs inhibits T to R quaternary structural change upon O2 binding. However, its absence from the β subunit in mutant and artificial Hbs simply enhances the O2 affinity of the α subunit. Accordingly, the inter-subunit interactions between α and β subunits are nonsymmetric but substantial for HbA to perform cooperative O2 binding.
Keywords: Cooperativity; Hemoglobin; Iron-histidine bond; Quaternary structure; Resonance Raman; Subunits.
© International Union for Pure and Applied Biophysics (IUPAB) and Springer-Verlag GmbH Germany, part of Springer Nature 2022.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures

References
-
- Adair GS. The hemoglobin system. VI. The oxygen dissociation curve of hemoglobin. J Biol Chem. 1925;63:529–545. doi: 10.1016/S0021-9258(18)85018-9. - DOI
-
- Balakrishnan G, Case MA, Pevsner A, Zhao X, Tengroth C, McLendon GL, Spiro TG. Time-resolved absorption and UV resonance Raman spectra reveal stepwise formation of T quaternary contacts in the allosteric pathway of hemoglobin. J Mol Biol. 2004;340:843–856. doi: 10.1016/j.jmb.2004.05.012. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

