Bulky cations greatly increase the turnover of a native hammerhead ribozyme
- PMID: 35528091
- PMCID: PMC9074697
- DOI: 10.1039/c9ra06797c
Bulky cations greatly increase the turnover of a native hammerhead ribozyme
Abstract
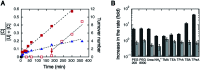
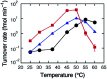
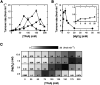
Methods to facilitate the catalytic turnover of ribozymes are required for advancing oligonucleotide-based technologies. This study examined tetraalkylammonium ions for their ability to increase the efficiency of catalytic turnover of a native hammerhead ribozyme. Kinetic analysis showed that large tetraalkylammonium ions significantly increased the turnover rate of the ribozyme and was much more effective than poly(ethylene glycol) (PEG) and urea. The magnitude of the rate increase depended on the concentrations of Mg2+ and tetrapentylammonium ions, and the rate was enhanced by more than 180-fold at the optimal concentrations of these salts. The results provide physical insights into interactions of ribozymes with large cationic molecules through electrostatic forces and steric hindrance.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Enhancement of the Catalytic Activity of Hammerhead Ribozymes by Organic Cations.Chembiochem. 2021 Sep 2;22(17):2721-2728. doi: 10.1002/cbic.202100280. Epub 2021 Jul 22. Chembiochem. 2021. PMID: 34240789
-
Existence of efficient divalent metal ion-catalyzed and inefficient divalent metal ion-independent channels in reactions catalyzed by a hammerhead ribozyme.Nucleic Acids Res. 2002 Jun 1;30(11):2374-82. doi: 10.1093/nar/30.11.2374. Nucleic Acids Res. 2002. PMID: 12034824 Free PMC article.
-
Hammerhead ribozyme targeting connective tissue growth factor mRNA blocks transforming growth factor-beta mediated cell proliferation.Exp Eye Res. 2004 Jun;78(6):1127-36. doi: 10.1016/j.exer.2004.01.012. Exp Eye Res. 2004. PMID: 15109919
-
Structural Simplicity and Mechanistic Complexity in the Hammerhead Ribozyme.Prog Mol Biol Transl Sci. 2018;159:177-202. doi: 10.1016/bs.pmbts.2018.07.006. Epub 2018 Sep 17. Prog Mol Biol Transl Sci. 2018. PMID: 30340787 Review.
-
Ribozymes: from mechanistic studies to applications in vivo.J Biochem. 1995 Aug;118(2):251-8. doi: 10.1093/oxfordjournals.jbchem.a124899. J Biochem. 1995. PMID: 8543555 Review.
Cited by
-
Hammerhead Ribozymes: Structural Insights, Catalytic Mechanisms, and Cutting-Edge Applications in Synthetic Biology.Int J Mol Sci. 2025 Jun 12;26(12):5624. doi: 10.3390/ijms26125624. Int J Mol Sci. 2025. PMID: 40565088 Free PMC article. Review.
-
Single Mutation in Hammerhead Ribozyme Favors Cleavage Activity with Manganese over Magnesium.Noncoding RNA. 2020 Mar 20;6(1):14. doi: 10.3390/ncrna6010014. Noncoding RNA. 2020. PMID: 32245091 Free PMC article.
References
-
- Barciszewska M. Z. Szymanski M. Wyszko E. Pas J. Rychlewski L. Barciszewski J. Mutat. Res. 2005;589:103–110. - PubMed
LinkOut - more resources
Full Text Sources

