Baicalin Attenuated A β 1-42-Induced Apoptosis in SH-SY5Y Cells by Inhibiting the Ras-ERK Signaling Pathway
- PMID: 35528169
- PMCID: PMC9068334
- DOI: 10.1155/2022/9491755
Baicalin Attenuated A β 1-42-Induced Apoptosis in SH-SY5Y Cells by Inhibiting the Ras-ERK Signaling Pathway
Abstract
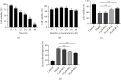
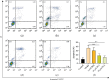
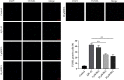
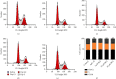
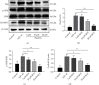
Alzheimer's disease (AD) is a serious neurodegenerative disease. It is widely believed that the accumulation of amyloid beta (Aβ) in neurons around neurofibrillary plaques is the main pathological characteristic of AD; however, the molecular mechanism underlying these pathological changes is not clear. Baicalin is a flavonoid extracted from the dry root of Scutellaria baicalensis Georgi. Studies have shown that baicalin exerts excellent anti-inflammatory and neuroprotective effects. In this study, an AD cell model was established by exposing SH-SY5Y cells to Aβ 1-42 and treating them with baicalin. Cell survival, cell cycle progression, and apoptosis were measured by MTT, flow cytometry, and immunofluorescence assays, respectively. The expression levels of Ras, ERK/ERK phosphorylation (p-ERK), and cyclin D1 were measured by Western blotting. In addition, whether the MEK activator could reverse the regulatory effect of baicalin on Ras-ERK signaling was investigated using Western blotting. We found that baicalin improved the survival, promoted the proliferation, and inhibited the apoptosis of SH-SY5Y cells after Aβ 1-42 treatment. Baicalin also ameliorated Aβ 1-42-induced cell cycle arrest at the S phase and induced apoptosis. Furthermore, baicalin inhibited the levels of Ras, p-ERK, and cyclin D1 induced by Aβ, and this effect could be reversed by the MEK activator. Therefore, we suggest that baicalin may regulate neuronal cell cycle progression and apoptosis in Aβ 1-42-treated SH-SY5Y cells by inhibiting the Ras-ERK signaling pathway. This study suggested that baicalin might be a useful therapeutic agent for senile dementia, especially AD.
Copyright © 2022 Zhenyan Song et al.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures

Similar articles
-
The Effects and Regulatory Mechanism of Flavonoids from Stems and Leaves of Scutellaria baicalensis Georgi in Promoting Neurogenesis and Improving Memory Impairment Mediated by the BDNF-ERK-CREB Signaling Pathway in Rats.CNS Neurol Disord Drug Targets. 2022;21(4):354-366. doi: 10.2174/1871527320666210827112048. CNS Neurol Disord Drug Targets. 2022. PMID: 34455975
-
Liraglutide prevents beta-amyloid-induced neurotoxicity in SH-SY5Y cells via a PI3K-dependent signaling pathway.Neurol Res. 2016 Apr;38(4):313-9. doi: 10.1080/01616412.2016.1145914. Epub 2016 Apr 23. Neurol Res. 2016. PMID: 27108910
-
Tβ4 ameliorates oxidative damage and apoptosis through ERK/MAPK and 5-HT1A signaling pathway in Aβ insulted SH-SY5Y cells.Life Sci. 2021 Nov 25:120178. doi: 10.1016/j.lfs.2021.120178. Online ahead of print. Life Sci. 2021. PMID: 34838849
-
Therapeutic Effects of Baicalin on Diseases Related to Gut-Brain Axis Dysfunctions.Molecules. 2023 Sep 7;28(18):6501. doi: 10.3390/molecules28186501. Molecules. 2023. PMID: 37764277 Free PMC article. Review.
-
The protective effects of baicalin for respiratory diseases: an update and future perspectives.Front Pharmacol. 2023 Mar 16;14:1129817. doi: 10.3389/fphar.2023.1129817. eCollection 2023. Front Pharmacol. 2023. PMID: 37007037 Free PMC article. Review.
Cited by
-
De novo design of a mechano-pharmaceutical screening platform against formation of individual beta-amyloid oligomers.Cell Rep Phys Sci. 2024 Dec 18;5(12):102336. doi: 10.1016/j.xcrp.2024.102336. Cell Rep Phys Sci. 2024. PMID: 40083584 Free PMC article.
-
Δ8-THC Protects against Amyloid Beta Toxicity Modulating ER Stress In Vitro: A Transcriptomic Analysis.Int J Mol Sci. 2023 Apr 2;24(7):6598. doi: 10.3390/ijms24076598. Int J Mol Sci. 2023. PMID: 37047608 Free PMC article.
-
Research Progress on Natural Plant Molecules in Regulating the Blood-Brain Barrier in Alzheimer's Disease.Molecules. 2023 Nov 16;28(22):7631. doi: 10.3390/molecules28227631. Molecules. 2023. PMID: 38005352 Free PMC article. Review.
-
The Neuroprotective Potential of Seed Extract from the Indian Trumpet Tree Against Amyloid Beta-Induced Toxicity in SH-SY5Y Cells.Int J Mol Sci. 2025 Jun 29;26(13):6288. doi: 10.3390/ijms26136288. Int J Mol Sci. 2025. PMID: 40650065 Free PMC article.
-
The potential of baicalin to enhance neuroprotection and mitochondrial function in a human neuronal cell model.Mol Psychiatry. 2024 Aug;29(8):2487-2495. doi: 10.1038/s41380-024-02525-5. Epub 2024 Mar 19. Mol Psychiatry. 2024. PMID: 38503930 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

