Antibody Response and Maternofetal Antibody Transfer in SARS-CoV-2-Positive Pregnant Women: A Multicenter Observational Study
- PMID: 35528188
- PMCID: PMC9076216
- DOI: 10.1055/a-1768-0415
Antibody Response and Maternofetal Antibody Transfer in SARS-CoV-2-Positive Pregnant Women: A Multicenter Observational Study
Abstract
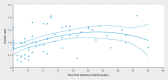
Introduction Awareness of SARS-CoV-2 infection in pregnant women and the potential risk for infection of their neonates is increasing. The aim of this study was to examine the immune status of affected women and evaluate the dynamics of placental antibody transfer. Materials and Methods The study included 176 women with SARS-CoV-2 infection during pregnancy who delivered between April 2020 and December 2021 at eight obstetric maternity sites. Demographic data, maternal and neonatal characteristics were summarized. Antibody testing for IgA and IgG in maternal blood sera and umbilical cord samples was evaluated and IgG transfer ratios were calculated. Values were related to the time of infection during pregnancy and birth. Results The percentage of IgG positive women increased from 29.0% (95% CI 23.8 - 37.8) at presentation with a positive PCR test result to 75.7% (95% CI 71.6 - 79.8), the percentage of IgG positive umbilical cord blood samples increased from 17.1% (95% CI 13.0 - 21.3) to 76.4% (95% CI 72.2 - 80.7) at more than six weeks after infection. Regression lines differed significantly between maternal and fetal IgG responses (p < 0.0001). Newborns react with a latency of about one week; umbilical cord blood antibody concentrations are highly correlated with maternal concentration levels (ρ = 0.8042; p < 0.0001). IgG transplacental transfer ratios were dependent on infection-to-birth interval. Two of the umbilical cord blood samples tested positive for IgA. Conclusions These findings confirm vertical SARS-CoV-2 transmission is rare; however, antibodies are transferred to the fetus soon after infection during pregnancy. Since transplacental antibody transfer might have a protective value for neonatal immunization this information may be helpful when counseling affected women.
Einleitung Das Wissen um die Auswirkungen von SARS-CoV-2-Infektionen in schwangeren Frauen und um das potenzielle Risiko einer Infektion ihrer neugeborenen Kinder wächst. Ziel dieser Studie war es, den Immunstatus von betroffenen Frauen und die Wechselwirkungen des plazentären Transfers von Antikörpern zu untersuchen. Material und Methoden Es wurden 176 Frauen, die während ihrer Schwangerschaft mit SARS-CoV-2-infiziert wurden und zwischen April 2020 und Dezember 2021 in einer von 8 Geburtskliniken entbanden, in die Studie aufgenommen. Demografische Daten und Charakteristika von Müttern und Neugeborenenen wurden aufgenommen. Die Ergebnisse von IgA- und IgG-Antikörpertests im mütterlichen Blut und Nabelschnurblut wurden evaluiert und die IgG-Transfer-Quotienten wurden berechnet. Die Werte wurden dem jeweiligen Zeitpunkt der Infektion während der Schwangerschaft und der Geburt zugeordnet. Ergebnisse Der Prozentsatz an IgG-positiven Frauen erhöhte sich von 29,0% (95%-KI 23,8 – 37,8) bei Vorliegen eines positiven PCR-Tests auf 75,7% (95%-KI 71,6 – 79,8), der Prozentsatz an IgG-positiven Blutproben von Nabelschnüren erhöhte sich von 17,1% (95%-KI 13,0 – 21,3) auf 76,4% (95%-KI 72,2 – 80,7) mehr als 6 Wochen nach der Infektion. Es gab signifikante Unterschiede zwischen den jeweilligen Regressionslinien der mütterlichen und der fötalen Immunantwort auf IgG (p < 0,0001). Neugeborenene reagierten mit eine Latenzzeit von ungefähr 1 Woche; Antikörperkonzentrationen im Nabelschnurblut korrelierten sehr stark mit den mütterlichen Konzentrationen (ρ = 0,8042; p < 0,0001). Der transplazentäre IgG-Transfer-Quotient hing von dem Zeitintervall zwischen Infektion und Geburt ab. Zwei der mittels Nabelschnurpunktion gewonnenen Blutproben waren IgA-positiv. Schlussfolgerungen Diese Ergebnisse bestätigen, dass vertikale Transmission von SARS-CoV-2 selten vorkommt; kurz nach der mütterlichen Infektion während der Schwangerschaft werden aber Antikörper zum Fötus transferiert. Da ein transplanzentärer Transfer von Antikörpern einen gewissen Schutz bei der Immunisierung des Neugeborenen bietet, könnte das eine nützliche Information bei der Beratung betroffener Frauen sein.
Keywords: COVID-19; SARS-CoV-2; antibody; pregnancy.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commecial purposes, or adapted, remixed, transformed or built upon. ( https://creativecommons.org/licenses/by-nc-nd/4.0/ ).
Conflict of interest statement
Conflict of Interest Sabine Enengl and Peter Oppelt received a grant for COVID-19 research from Johannes Kepler University Linz. All other authors declare that they have no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

