N-((1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-2-methylene-3-oxo-olean-12-en-28-amide Induces Apoptosis in Human Breast Cancer Cells by Stimulating Oxidative Stress and Inhibiting the Notch-Akt Signaling Pathway
- PMID: 35528507
- PMCID: PMC9068303
- DOI: 10.1155/2022/8123120
N-((1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-2-methylene-3-oxo-olean-12-en-28-amide Induces Apoptosis in Human Breast Cancer Cells by Stimulating Oxidative Stress and Inhibiting the Notch-Akt Signaling Pathway
Abstract
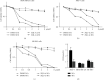
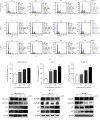
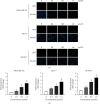
Breast cancer is of the leading causes of cancer-related deaths and the most frequently diagnosed cancer among females worldwide. Despite advancements in breast cancer therapy, the disease eventually progresses in most patients because of de novo or secondary resistance. Thus, discovering novel drugs with high effectiveness and low toxicity for systemic therapy is essential. In this study, we investigated whether a new oleanolic derivative N-((1-(4-fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-2-methylene-3-oxo-olean-12-en-28-amide (ZQL-4c) exhibits potential anticancer effects against breast cancer. We determined that ZQL-4c strongly inhibited cell proliferation and invasion and induced G2/M phase arrest and apoptosis in breast cancer cells. We then found that ZQL-4c induced the production of reactive oxygen species (ROS). We then found that ZQL-4c significantly inhibited Notch-AKT signaling pathways that are related to oxidative stress. Taken together, this study is the first to show that ZQL-4c can significantly suppress the growth and invasion of breast cancer by blocking Notch-Akt signaling pathways, which are mainly regulated by ROS-mediated oxidative stress. Thus, ZQL-4c might be considered a novel and potential anticancer drug for breast cancer treatment.
Copyright © 2022 Xiaorui Li et al.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Wu C., Qiu S., Liu P., Ge Y., Gao X. Rhizoma Amorphophalli inhibits TNBC cell proliferation, migration, invasion and metastasis through the PI3K/Akt/mTOR pathway. Journal of ethnopharmacology . 2018;211:89–100. - PubMed
-
- Waks A. G., Winer E. P. Breast cancer treatment: a Review. JAMA . 2019;321(3):288–300. - PubMed
-
- Nahta R., O'Regan R. M. Evolving strategies for overcoming resistance to HER2-directed therapy: targeting the PI3K/Akt/mTOR pathway. Clinical breast cancer . 2010;10 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

