Automated Retinal Vein Cannulation on Silicone Phantoms Using Optical-Coherence-Tomography-Guided Robotic Manipulations
- PMID: 35528629
- PMCID: PMC9075181
- DOI: 10.1109/tmech.2020.3045875
Automated Retinal Vein Cannulation on Silicone Phantoms Using Optical-Coherence-Tomography-Guided Robotic Manipulations
Abstract
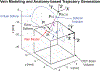
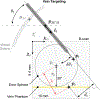
Retinal vein occlusion is one of the most common causes of vision loss, occurring when a blood clot or other obstruction occludes a retinal vein. A potential remedy for retinal vein occlusion is retinal vein cannulation, a surgical procedure that involves infusing the occluded vein with a fibrinolytic drug to restore blood flow through the vascular lumen. This work presents an image-guided robotic system capable of performing automated cannulation on silicone retinal vein phantoms. The system is integrated with an optical coherence tomography probe and camera to provide visual feedback to guide the robotic system. Through automation, the developed system targets a vein phantom to within 20 μm and automatically cannulates and infuses the vascular lumen with dyed water. The system was evaluated through 30 experimental trials and shown to be capable of performing automated cannulation of retinal vein phantoms with no reported cases of failure.
Figures
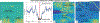


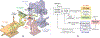
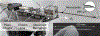
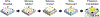





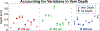
References
-
- Weiss JN, “Retinal surgery for treatment of central retinal vein occlusion,” Ophthalmic Surgery, Lasers and Imaging Retina, vol. 31, no. 2, pp. 162–165, 2000. - PubMed
-
- Ouyang Y et al., “Retinal vessel diameter measurements by spectral domain optical coherence tomography,” Graefe’s Archive for Clinical and Experimental Ophthalmology, vol. 253, no. 4, pp. 499–509, 2015. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
