Thermostability of protein nanocages: the effect of natural extra peptide on the exterior surface
- PMID: 35528680
- PMCID: PMC9069879
- DOI: 10.1039/c9ra04785a
Thermostability of protein nanocages: the effect of natural extra peptide on the exterior surface
Abstract
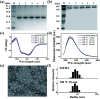
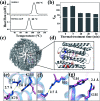
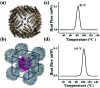
Protein nanocages have been used as functional bio-templates for the synthesis or organization of nanomaterials. However, the stability of these protein nanocages is nonideal, which limits their applications. Herein, we characterized the high thermal stability of plant ferritin, soybean seed H-2 ferritin (SSFH-2), the melting point (T m) of which is 106 °C. We demonstrated that the hyperthermostability of SSFH-2 is derived from extra peptides (EP) located on its outer surface. Indeed, removal of the EP domains resulted in a dramatic decrease in T m to 88 °C. Similar to EP-deleted plant ferritin, human H-chain ferritin (HuHF) has a T m of 82 °C. Excitingly, the graft of the EP domain on the exterior surface of HuHF pronouncedly improved its T m to 103 °C, which represents a simple, efficient approach to the construction of protein architectures with high stability. The remarkable stability of protein nanocages will greatly facilitate their application as robust biotemplates in the field of nanoscience.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Re-designing ferritin nanocages for mercuric ion detection.Analyst. 2019 Sep 23;144(19):5890-5897. doi: 10.1039/c9an01110b. Analyst. 2019. PMID: 31497803
-
Hyperthermostability of prawn ferritin nanocage facilitates its application as a robust nanovehicle for nutraceuticals.Int J Biol Macromol. 2021 Nov 30;191:152-160. doi: 10.1016/j.ijbiomac.2021.09.067. Epub 2021 Sep 20. Int J Biol Macromol. 2021. PMID: 34547309
-
Structural Insights for the Stronger Ability of Shrimp Ferritin to Coordinate with Heavy Metal Ions as Compared to Human H-Chain Ferritin.Int J Mol Sci. 2021 Jul 23;22(15):7859. doi: 10.3390/ijms22157859. Int J Mol Sci. 2021. PMID: 34360624 Free PMC article.
-
Redesign of protein nanocages: the way from 0D, 1D, 2D to 3D assembly.Chem Soc Rev. 2021 Mar 21;50(6):3957-3989. doi: 10.1039/d0cs01349h. Epub 2021 Feb 15. Chem Soc Rev. 2021. PMID: 33587075 Review.
-
Ferritin nanocages: A biological platform for drug delivery, imaging and theranostics in cancer.Pharmacol Res. 2016 May;107:57-65. doi: 10.1016/j.phrs.2016.03.002. Epub 2016 Mar 9. Pharmacol Res. 2016. PMID: 26968122 Review.
Cited by
-
Hyperthermostable recombinant human heteropolymer ferritin derived from a novel plasmid design.Protein Sci. 2023 Jan;32(1):e4543. doi: 10.1002/pro.4543. Protein Sci. 2023. PMID: 36519270 Free PMC article.
-
The Change in the Structure and Functionality of Ferritin during the Production of Pea Seed Milk.Foods. 2022 Feb 16;11(4):557. doi: 10.3390/foods11040557. Foods. 2022. PMID: 35206035 Free PMC article.
-
A Novel Hyperthermostable Recombinant Protein Nanocage.Iran Biomed J. 2022 Nov 1;26(6):426-39. doi: 10.52547/ibj.3839. Iran Biomed J. 2022. PMID: 36437775 Free PMC article.
-
Simultaneous Organic and Inorganic Host-Guest Chemistry within Pillararene-Protein Cage Frameworks.Chemistry. 2022 Feb 19;28(11):e202104341. doi: 10.1002/chem.202104341. Epub 2022 Feb 2. Chemistry. 2022. PMID: 35043998 Free PMC article.
-
Functional Characterization of Salmonella Typhimurium Encoded YciF, a Domain of Unknown Function (DUF892) Family Protein, and Its Role in Protection during Bile and Oxidative Stress.J Bacteriol. 2023 Jul 25;205(7):e0005923. doi: 10.1128/jb.00059-23. Epub 2023 Jun 27. J Bacteriol. 2023. PMID: 37367303 Free PMC article.
References
LinkOut - more resources
Full Text Sources

