Salidroside enhances the anti-cancerous effect of imatinib on human acute monocytic leukemia via the induction of autophagy-related apoptosis through AMPK activation
- PMID: 35528698
- PMCID: PMC9070041
- DOI: 10.1039/c9ra01683j
Salidroside enhances the anti-cancerous effect of imatinib on human acute monocytic leukemia via the induction of autophagy-related apoptosis through AMPK activation
Retraction in
-
Retraction: Salidroside enhances the anti-cancerous effect of imatinib on human acute monocytic leukemia via the induction of autophagy-related apoptosis through AMPK activation.RSC Adv. 2024 Apr 29;14(20):13957. doi: 10.1039/d4ra90050b. eCollection 2024 Apr 25. RSC Adv. 2024. PMID: 38686282 Free PMC article.
Abstract
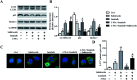
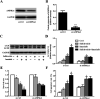
As the typical tyrosine kinase inhibitor, imatinib has been the first-line antineoplastic agent for both chronic myeloid leukemia and acute lymphoblastic leukemia. However, a large number of patients are still resistant to the benefits of imatinib, and they have a dissatisfactory prognosis. Salidroside, a compound that is extracted from natural plants, has been reported to have an excellent anticancer effect and few side effects. In the present study, we have developed a new combination therapy strategy of salidroside and imatinib for combating the growth of acute lymphoblastic leukemia. As demonstrated by the anti-proliferation assay, salidroside exhibited excellent cytotoxicity against myeloid leukemia cells. Moreover, cells treated by the combination therapy of salidroside and imatinib displayed a clear lower growth rate than cells only treated by imatinib, indicating that salidroside has a positive effect on enhancing the cytotoxicity of imatinib against leukemia cells. Subsequently, the underlying mechanisms were investigated. The results revealed that autophagy marker proteins in leukemia cells, including LC3, p62, and Beclin1, displayed a significant expression change after treating them with salidroside plus imatinib, with the levels of LC3 and Beclin1 dramatically increasing while the expression of p62 was significantly decreased. Moreover, an obvious down-regulation of p-PI3K, p-AKT and p-mTOR expression levels in leukemia cells after treatment with salidroside plus imatinib suggested that the PI3K/mTOR pathway plays an important role in the process of cell apoptosis induced by salidroside or imatinib. Further studies showed that pre-incubating the cells with an autophagy inhibitor dramatically inhibited the ability of imatinib to induce autophagy, but did not inhibit the ability of salidroside. The underlying causes were subsequently explored and the results showed that silencing AMPKα1, the most important regulator of autophagy, dramatically attenuates the ability of salidroside to induce cell apoptosis. These results together indicated that salidroside enhances the cytotoxicity of imatinib on acute monocytic leukemia via the induction of autophagy-related apoptosis through AMPK activation. The unique advantages of combination therapy were further confirmed by in vivo experiments, with the tumor-bearing cells treated with salidroside plus imatinib achieving the best anti-tumor effect.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

