Methods for direct C(sp2)-H bonds azidation
- PMID: 35528700
- PMCID: PMC9069887
- DOI: 10.1039/c9ra04534a
Methods for direct C(sp2)-H bonds azidation
Abstract
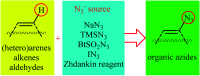
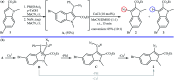
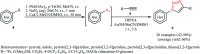
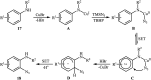
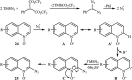
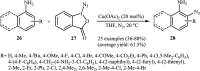
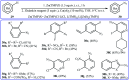
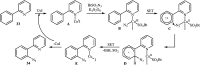
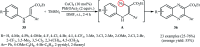
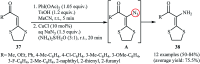
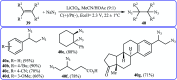
Direct functionalization of C-H bonds has attracted great attention in recent years from the perspectives of atom and step economy. In this context, a variety of processes have been developed for the construction of synthetically and biologically important organic azides through the oxidative C-H bonds azidation. In this review, we have summarized recent progress in the direct azidation of C(sp2)-H bonds. The review is divided into three major sections: (i) direct azidation of aromatic C-H bonds; (ii) direct azidation of olefinic C-H bonds; and (iii) direct azidation of aldehydic C-H bonds. Mechanistic aspects of the reactions are considered and discussed in detail.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




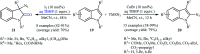




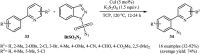


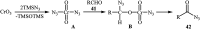




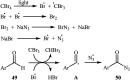



Similar articles
-
A Metal-Free Oxidative Azidation Method for Selective Azidation of C(sp3)-H Bonds and Diazidation of Alkenes.Org Lett. 2025 May 30;27(21):5373-5378. doi: 10.1021/acs.orglett.5c01241. Epub 2025 May 21. Org Lett. 2025. PMID: 40396439
-
Dirhodium(II)-Catalyzed C(sp2 )-H Azidation of Benzaldehydes.Chemistry. 2020 May 26;26(30):6805-6811. doi: 10.1002/chem.201905855. Epub 2020 Apr 28. Chemistry. 2020. PMID: 32045045
-
Manganese-catalyzed late-stage aliphatic C-H azidation.J Am Chem Soc. 2015 Apr 29;137(16):5300-3. doi: 10.1021/jacs.5b01983. Epub 2015 Apr 14. J Am Chem Soc. 2015. PMID: 25871027
-
Recent progress in the oxidative coupling of unactivated Csp3-H bonds with other C-H bonds.Chem Commun (Camb). 2021 Dec 9;57(98):13288-13296. doi: 10.1039/d1cc04802c. Chem Commun (Camb). 2021. PMID: 34825675 Review.
-
A walk around the application of nanocatalysts for cross-dehydrogenative coupling of C-H bonds.RSC Adv. 2019 Dec 16;9(71):41684-41702. doi: 10.1039/c9ra08752d. eCollection 2019 Dec 13. RSC Adv. 2019. PMID: 35557874 Free PMC article. Review.
Cited by
-
Direct halosulfonylation of alkynes: an overview.RSC Adv. 2021 Oct 13;11(53):33447-33460. doi: 10.1039/d1ra03443j. eCollection 2021 Oct 8. RSC Adv. 2021. PMID: 35497552 Free PMC article. Review.
-
N-Fluorobenzenesulfonimide: a useful and versatile reagent for the direct fluorination and amination of (hetero)aromatic C-H bonds.RSC Adv. 2020 Apr 29;10(28):16756-16768. doi: 10.1039/d0ra00324g. eCollection 2020 Apr 23. RSC Adv. 2020. PMID: 35498838 Free PMC article. Review.
-
Progress and recent trends in the direct selenocyanation of (hetero)aromatic C-H bonds.RSC Adv. 2021 Jun 24;11(36):22305-22316. doi: 10.1039/d1ra01035b. eCollection 2021 Jun 21. RSC Adv. 2021. PMID: 35480846 Free PMC article. Review.
-
Recent trends in the direct oxyphosphorylation of C-C multiple bonds.RSC Adv. 2020 Dec 23;11(1):470-483. doi: 10.1039/d0ra08074h. eCollection 2020 Dec 21. RSC Adv. 2020. PMID: 35423055 Free PMC article. Review.
-
Hydroxysulfonylation of alkenes: an update.RSC Adv. 2021 Jun 18;11(35):21651-21665. doi: 10.1039/d1ra00513h. eCollection 2021 Jun 15. RSC Adv. 2021. PMID: 35478812 Free PMC article. Review.
References
-
- Amblard F. Cho J. H. Schinazi R. F. Chem. Rev. 2009;109:4207–4220. doi: 10.1021/cr9001462. - DOI - PMC - PubMed
- Bräse S. Gil C. Knepper K. Zimmermann V. Angew. Chem., Int. Ed. 2005;44:5188–5240. doi: 10.1002/anie.200400657. - DOI - PubMed
- Cramer S. A. Jenkins D. M. J. Am. Chem. Soc. 2011;133:19342–19345. doi: 10.1021/ja2090965. - DOI - PubMed
- Huang D. Yan G. Adv. Synth. Catal. 2017;359:1600–1619. doi: 10.1002/adsc.201700103. - DOI
- Saeidian H. Sadighian H. Abdoli M. Sahandi M. J. Mol. Struct. 2017;1131:73–78. doi: 10.1016/j.molstruc.2016.11.027. - DOI
-
- Fazio F. Wong C.-H. Tetrahedron Lett. 2003;44:9083–9085. doi: 10.1016/j.tetlet.2003.10.054. - DOI
- Xie S. Zhang Y. Ramström O. Yan M. Chem. Sci. 2016;7:713–718. doi: 10.1039/C5SC03510D. - DOI - PMC - PubMed
- Zhang F.-L. Zhu X. Chiba S. Org. Lett. 2015;17:3138–3141. doi: 10.1021/acs.orglett.5b01458. - DOI - PubMed
-
- Ramesha A. Bhat S. Chandrasekaran S. J. Org. Chem. 1995;60:7682–7683. doi: 10.1021/jo00128a048. - DOI
- Benati L. Bencivenni G. Leardini R. Nanni D. Minozzi M. Spagnolo P. Scialpi R. Zanardi G. Org. Lett. 2006;8:2499–2502. doi: 10.1021/ol0606637. - DOI - PubMed
- Lee J. H. Gupta S. Jeong W. Rhee Y. H. Park J. Angew. Chem., Int. Ed. 2012;51:10851–10855. doi: 10.1002/anie.201204483. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

