Nitrogen aeration alters the spatial distribution and metal adsorption of extracellular polymeric substances in waste-activated sludge
- PMID: 35528892
- PMCID: PMC9073697
- DOI: 10.1039/c9ra07576c
Nitrogen aeration alters the spatial distribution and metal adsorption of extracellular polymeric substances in waste-activated sludge
Abstract
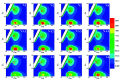
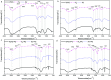
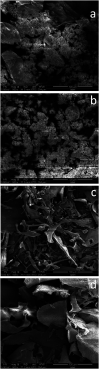
Extracellular polymeric substances (EPS) extracted from waste-activated sludge (WAS) have the potential to remove heavy metal ions from wastewater; both the spatial distribution and metal adsorption of EPS from WAS after nitrogen aeration were systematically investigated in this study. Compared with air aeration, nitrogen aeration significantly improved the contents of proteins (PN) and polysaccharides (PS) in the Slime-EPS (S-EPS) and loosely-bound EPS (LB-EPS), significantly increased the PS content, and slightly increased the PN content in the tightly-bound EPS (TB-EPS). The variations in the fluorescence intensities (FI) of the peaks I and II for the S-EPS, LB-EPS and TB-EPS were basically consistent with the abovementioned variations in the concentrations of these EPS. Notably, nitrogen aeration dramatically improved the content of protein-like substances in the LB-EPS. For the same aeration time, the Pb2+ reclamation rates obtained by the LB-EPS extracted from the nitrogen-aerated WAS were much higher than those achieved by the LB-EPS extracted from the air-aerated WAS. The FTIR analyses further indicated that nitrogen aeration improved the contents of the functional groups, especially -OH, -COOH and -NH2, responsible for binding heavy metals, in both the LB-EPS and TB-EPS. The SEM analyses verified that the nitrogen scours contributed to the EPS release, and Pb2+ reclamation was achieved via the attachment of Pb2+ onto the edge of the EPS. The influences of the nitrogen aeration on the spatial distribution and metal adsorption of the EPS in WAS were revealed for the first time in this study. Thus, this study lays the foundation for the application of nitrogen aeration in the resource utilization of WAS.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Guibaud G. Comte S. Bordas F. Baudu M. Process Biochem. 2005;40:661–668. doi: 10.1016/j.procbio.2004.01.059. - DOI
LinkOut - more resources
Full Text Sources

