Melanin-mimetic multicolor and low-toxicity hair dye
- PMID: 35528905
- PMCID: PMC9073633
- DOI: 10.1039/c9ra07466j
Melanin-mimetic multicolor and low-toxicity hair dye
Abstract
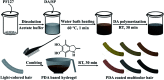
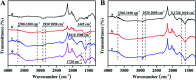
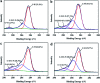
Most commercial permanent hair dyeing technologies are based on the oxidative process of p-phenylenediamine and its derivative materials. However, concerns about their toxicological issues have been raised throughout the years. Herein, we report an innovative surface coloration strategy for fabricating melanin-mimetic multicolor and low-toxicity hair dyes through sodium periodate-induced rapid deposition of eumelanin-like polydopamine (PDA) and pheomelanin-like PDA/cysteine co-deposited coatings on the hair surface. The color and morphology of the resulting hair were characterized in detail by several spectroscopy methods and the possible mechanism for the multi-coloring effects and structural differences of the melanin-mimetic coating was proposed. Our strategy eliminates the use of toxic dye precursors or organic solvents, and the favorable safety of the PDA-based formulations is demonstrated. The fabricated dyes can be applied to hair simply by combing, resulting in uniform multi-coloring effects within a short time. Furthermore, the melanin-mimetic hair dyes have excellent durability and ultraviolet protection performance. This work provides a facile and versatile methodology to develop the next generation of safe, sustainable and multicolor hair dyes and pave new avenues for advancing the field of surface coloration, nanoreactors, nanogenerators, energy storage materials and biomimetic sensing devices.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Boga C. Delpivo C. Ballarin B. Morigi M. Galli S. Micheletti G. Tozzi S. Dyes Pigm. 2013;97:9–18. doi: 10.1016/j.dyepig.2012.11.020. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

