Long-Term High-Temperature Stress Impacts on Embryo and Seed Development in Brassica napus
- PMID: 35528932
- PMCID: PMC9075611
- DOI: 10.3389/fpls.2022.844292
Long-Term High-Temperature Stress Impacts on Embryo and Seed Development in Brassica napus
Abstract
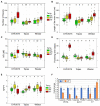
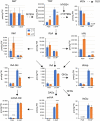
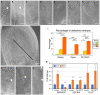
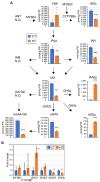
Brassica napus (rapeseed) is the second most important oilseed crop worldwide. Global rise in average ambient temperature and extreme weather severely impact rapeseed seed yield. However, fewer research explained the phenotype changes caused by moderate-to-high temperatures in rapeseed. To investigate these events, we determined the long-term response of three spring cultivars to different temperature regimes (21/18°C, 28/18°C, and 34/18°C) mimicking natural temperature variations. The analysis focused on the plant appearance, seed yield, quality and viability, and embryo development. Our microscopic observations suggest that embryonic development is accelerated and defective in high temperatures. Reduced viable seed yield at warm ambient temperature is due to a reduced fertilization rate, increased abortion rate, defective embryonic development, and pre-harvest sprouting. Reduced auxin levels in young seeds and low ABA and auxin levels in mature seeds may cause embryo pattern defects and reduced seed dormancy, respectively. Glucosinolates and oil composition measurements suggest reduced seed quality. These identified cues help understand seed thermomorphogenesis and pave the way to developing thermoresilient rapeseed.
Keywords: Brassica napus; embryo development; high temperatures; hormonal profiling; oil content; seed development; thermomorphogenesis.
Copyright © 2022 Mácová, Prabhullachandran, Štefková, Spyroglou, Pěnčík, Endlová, Novák and Robert.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Aksouh-Harradj N. M., Campbell L. C., Mailer R. J. (2006). Canola response to high and moderately high temperature stresses during seed maturation. Can. J. Plant Sci. 86, 967–980. doi: 10.4141/p05-130 - DOI
-
- Andersen C. L., Jensen J. L., Ørntoft T. F. (2004). Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 64, 5245–5250. doi: 10.1158/0008-5472.can-04-0496, PMID: - DOI - PubMed
-
- Angadi S. V., Cutforth H. W., Miller P. R., McConkey B. G., Entz M. H., Brandt S. A., et al. (2000). Response of three brassica species to high temperature stress during reproductive growth. Can. J. Plant Sci. 80, 693–701. doi: 10.4141/P99-152 - DOI
-
- Annisa A., Chen S., Turner N. C., Cowling W. A. (2013). Genetic variation for heat tolerance during the reproductive phase in Brassica rapa. J. Agron. Crop Sci. 199, 424–435. doi: 10.1111/jac.12034 - DOI
LinkOut - more resources
Full Text Sources

