Redefinition of Fatty Liver Disease from NAFLD to MAFLD through the Lens of Drug Development and Regulatory Science
- PMID: 35528969
- PMCID: PMC9039717
- DOI: 10.14218/JCTH.2021.00408
Redefinition of Fatty Liver Disease from NAFLD to MAFLD through the Lens of Drug Development and Regulatory Science
Abstract
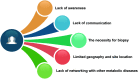
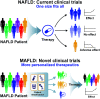
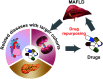
Metabolic (dysfunction)-associated fatty liver disease (MAFLD) affects a third of the population and is a leading cause of liver-related death. Since no effective treatments exist, novel approaches to drug development are required. Unfortunately, outdated terminology and definitions of the disease are hampering efforts to develop new drugs and treatments. An international consensus panel has put forth an influential proposal for the disease to be renamed from nonalcoholic fatty liver disease (NAFLD) to MAFLD, including a proposal for how the disease should be diagnosed. As allies with the many stakeholders in MAFLD care-including patients, patients' advocates, clinicians, researchers, nurse and allied health groups, regional societies, and others-we are aware of the negative consequences of the NAFLD term and definition. We share the sense of urgency for change and will act in new ways to achieve our goals. Although there is much work to be done to overcome clinical inertia and reverse worrisome recent trends, the MAFLD initiative provides a firm foundation to build on. It provides a roadmap for moving forward toward more efficient care and affordable, sustainable drug and device innovation in MAFLD care. We hope it will bring promising new opportunities for a brighter future for MAFLD care and improve care and outcomes for patients of one of the globe's largest and costliest public health burdens. From this viewpoint, we have revisited this initiative through the perspectives of drug development and regulatory science.
Keywords: Fatty liver disease; Fibrosis; Liver; MAFLD; NAFLD; NASH.
© 2022 Authors.
Conflict of interest statement
YF has been an editorial board member of Journal of Clinical and Translational Hepatology since 2021. The other authors have no conflict of interests related to this publication. The views presented in this article do not necessarily reflect those of the USA Food and Drug Administration. Any mention of commercial products is for clarification and is not intended as an endorsement.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
