Unraveling the Impact of pH on the Crystallization of Pharmaceutical Proteins: A Case Study of Human Insulin
- PMID: 35529069
- PMCID: PMC9073949
- DOI: 10.1021/acs.cgd.1c01463
Unraveling the Impact of pH on the Crystallization of Pharmaceutical Proteins: A Case Study of Human Insulin
Abstract
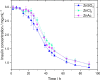
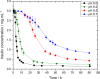
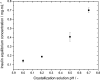
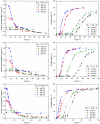
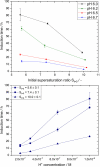
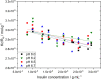
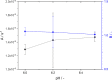
One of the most crucial parameters in protein crystallization is pH, as it governs the protein's electrostatic interactions. However, the fundamental role of pH on crystallization still remains unknown. Here, we systematically investigated the crystallization of human insulin (isoelectric point 5.3) at various pHs between 6.0 and 6.7 at different supersaturation ratios, up to 20.9. Our results demonstrate that the pH has an opposing effect on solubility and nucleation rate as a shift in pH toward a more basic milieu increases the solubility by 5-fold while the onset of nucleation was accelerated by a maximum of 8.6-fold. To shed light on this opposing effect, we evaluated the protein-protein interactions as a function of pH by measuring the second virial coefficient and hydrodynamic radius and showed that a change in pH of less than one unit has no significant impact on the protein-protein interactions. As it is widely understood that the increase in protein solubility as a function of pH is due to the increase in the repulsive electrostatic interactions, we have demonstrated that the increase in insulin solubility and decrease in the onset of nucleation are independent of the protein-protein interactions. We hypothesize that it is the electrostatic interactions between both ions and solvent molecules and the protein residues that are governing the crystallization of human insulin. The findings of this study will be of crucial importance for the design of novel crystallization pathways.
© 2022 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
