Formation of Salts and Molecular Ionic Cocrystals of Fluoroquinolones and α,ω-Dicarboxylic Acids
- PMID: 35529070
- PMCID: PMC9073931
- DOI: 10.1021/acs.cgd.1c01509
Formation of Salts and Molecular Ionic Cocrystals of Fluoroquinolones and α,ω-Dicarboxylic Acids
Abstract
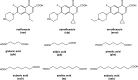
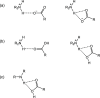
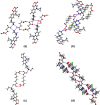
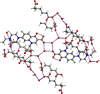
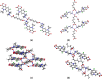
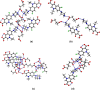
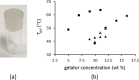
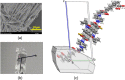
The cocrystallization of the fluoroquinolones ciprofloxacin (cip), norfloxacin (nor), and enrofloxacin (enro) with the α,ω-dicarboxylic acids glutaric acid (glu), adipic acid (adi), pimelic acid (pim), suberic acid (sub), azeliac acid (az), and sebacic acid (seb) resulted in 27 new molecular salts and ternary molecular ionic cocrystals of compositions A+B-, A2 +B2-, A2 +B2-B, and A+B-A. Depending on the solvent, different stoichiomorphs, solvates, or polymorphs were obtained. All salts and cocrystals contain the robust R2NH2 +...-OOC or R3NH+...-OOC synthon but have different supramolecular ring motifs. Moderate solubility enhancements over the parent fluoroquinolones were observed. Salts in the ratio of 1:1 and 2:1 were also prepared by ball-milling. The milled sample nor/az (1:1) was shown to gel the GRAS (generally recognized as safe) solvent propylene glycol, and enro/sub (1:1) was shown to gel both propylene glycol and water. Dynamic rheology measurements confirmed that nor/az and enro/sub behave like viscoelastic materials and supramolecular gels.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Salt and cocrystals of sildenafil with dicarboxylic acids: solubility and pharmacokinetic advantage of the glutarate salt.Mol Pharm. 2013 Dec 2;10(12):4687-97. doi: 10.1021/mp400516b. Epub 2013 Nov 8. Mol Pharm. 2013. PMID: 24168322
-
Differences in Coformer Interactions of the 2,4-Diaminopyrimidines Pyrimethamine and Trimethoprim.Cryst Growth Des. 2022 May 4;22(5):3163-3173. doi: 10.1021/acs.cgd.2c00035. Epub 2022 Apr 8. Cryst Growth Des. 2022. PMID: 35529062 Free PMC article.
-
Fluoroquinolone Amorphous Polymeric Salts and Dispersions for Veterinary Uses.Pharmaceutics. 2019 Jun 9;11(6):268. doi: 10.3390/pharmaceutics11060268. Pharmaceutics. 2019. PMID: 31181834 Free PMC article.
-
Multicomponent Pharmaceutical Cocrystals: A Novel Approach for Combination Therapy.Mini Rev Med Chem. 2018;18(14):1160-1167. doi: 10.2174/1389557518666180305163613. Mini Rev Med Chem. 2018. PMID: 29512461 Review.
-
Metabolic engineering for the production of dicarboxylic acids and diamines.Metab Eng. 2020 Mar;58:2-16. doi: 10.1016/j.ymben.2019.03.005. Epub 2019 Mar 21. Metab Eng. 2020. PMID: 30905694 Review.
Cited by
-
Co-crystal of nadifloxacin with oxalic acid.Acta Crystallogr E Crystallogr Commun. 2023 Mar 10;79(Pt 4):319-322. doi: 10.1107/S2056989023002244. eCollection 2023 Mar 1. Acta Crystallogr E Crystallogr Commun. 2023. PMID: 37057007 Free PMC article.
-
Mechanochemically Induced Solid-State Transformations of Levofloxacin.Mol Pharm. 2024 Jun 3;21(6):2838-2853. doi: 10.1021/acs.molpharmaceut.4c00008. Epub 2024 Apr 25. Mol Pharm. 2024. PMID: 38662637 Free PMC article.
-
Pharmaceutical cocrystal of antibiotic drugs: A comprehensive review.Heliyon. 2022 Nov 30;8(12):e11872. doi: 10.1016/j.heliyon.2022.e11872. eCollection 2022 Dec. Heliyon. 2022. PMID: 36478792 Free PMC article. Review.
-
The Benefits and Challenges of Antibiotics-Non-Steroidal Anti-Inflammatory Drugs Non-Covalent Reaction.Molecules. 2023 Apr 23;28(9):3672. doi: 10.3390/molecules28093672. Molecules. 2023. PMID: 37175082 Free PMC article. Review.
References
-
- Prabodh Chander S.; Ankit J.; Sander J. Fluoroquinolone antibacterials: a review on chemistry, microbiology and therapeutic prospects. Acta Pol. Pharm. 2009, 66, 587–604. - PubMed
-
- Pudipeddi M.; Serajuddin A. T. M.; Grant D. J. W.; Stahl P. H.. Handbook of Pharmaceutical Salts, Properties, Selection and Use; Stahl P. H.; Wermuth C. G., Eds.; Wiley-VCH: Weinheim, 2002; pp 19–40.
LinkOut - more resources
Full Text Sources
