Effect of the coexistence of albumin and H2O2 on the corrosion of biomedical cobalt alloys in physiological saline
- PMID: 35529113
- PMCID: PMC9073266
- DOI: 10.1039/c9ra05699h
Effect of the coexistence of albumin and H2O2 on the corrosion of biomedical cobalt alloys in physiological saline
Abstract
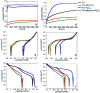
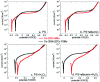
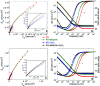
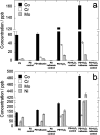
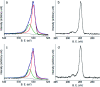
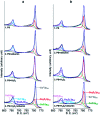
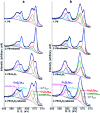
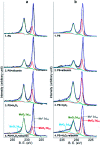
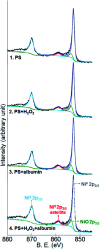
The corrosion of Co-28Cr-6Mo and Co-35Ni-20Cr-10Mo, as biomedical alloys, has been investigated for effects of typical species (albumin and H2O2) in physiological saline, with their coexistence explored for the first time. Electrochemical and long term immersion tests were carried out. It was found that Co alloys were not sensitive to the presence of albumin alone, which slightly promoted anodic dissolution of Co-35Ni-20Cr-10Mo without noticeably affecting Co-28Cr-6Mo and facilitated oxide film dissolution on both alloys. H2O2 led to a clear drop in corrosion resistance, favouring metal release and surface oxide formation and inducing much thicker but less compact oxide films for both alloys. The coexistence of both species resulted in the worst corrosion resistance and most metal release, while the amount and composition of surface oxide remained at a similar level as in the absence of both. The effect of H2O2 inducing low compactness of surface oxides should prevail on deciding the poor corrosion protection ability of passive film, while albumin simultaneously promoted dissolution or inhibited formation of oxides due to H2O2. Corrosion resistance was consistently lower for Co-35Ni-20Cr-10Mo under each condition, the only alloy where the synergistic effect of both species was clearly demonstrated. This work suggests that the complexity of the environment must be considered for corrosion resistance evaluation of biomedical alloys.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Comparison of the electrochemical behavior of CoCrWNi, CoCrFeNiMo, and CoNiCrMo alloys.J Biomed Mater Res B Appl Biomater. 2020 Oct;108(7):2971-2980. doi: 10.1002/jbm.b.34627. Epub 2020 May 9. J Biomed Mater Res B Appl Biomater. 2020. PMID: 32386114
-
The effect of serum proteins on the electrochemical behavior of CoNiCrMo.J Biomed Mater Res B Appl Biomater. 2023 Apr;111(4):775-781. doi: 10.1002/jbm.b.35188. Epub 2022 Nov 5. J Biomed Mater Res B Appl Biomater. 2023. PMID: 36333977
-
A synergistic effect of albumin and H₂O₂ accelerates corrosion of Ti6Al4V.Acta Biomater. 2015 Oct;26:355-65. doi: 10.1016/j.actbio.2015.07.046. Epub 2015 Jul 31. Acta Biomater. 2015. PMID: 26238758
-
Comparative analysis of corrosion resistance between beta titanium and Ti-6Al-4V alloys: A systematic review.J Trace Elem Med Biol. 2020 Dec;62:126618. doi: 10.1016/j.jtemb.2020.126618. Epub 2020 Jul 9. J Trace Elem Med Biol. 2020. PMID: 32663743
-
Metal release from stainless steel in biological environments: A review.Biointerphases. 2015 Mar 29;11(1):018901. doi: 10.1116/1.4934628. Biointerphases. 2015. PMID: 26514345 Review.
Cited by
-
Interaction pathways of implant metal localized corrosion and macrophage inflammatory reactions.Bioact Mater. 2023 Aug 27;31:355-367. doi: 10.1016/j.bioactmat.2023.08.017. eCollection 2024 Jan. Bioact Mater. 2023. PMID: 37663618 Free PMC article.
References
-
- Pramanik S. Agarwal A. K. Rai K. N. Trends Biomater. Artif. Organs. 2005;19:15–26.
-
- Liu Y. P. Gilbert J. L. Electrochim. Acta. 2018;262:252–263. doi: 10.1016/j.electacta.2017.12.151. - DOI
LinkOut - more resources
Full Text Sources

