Recent advances in direct trifluoromethylation of olefinic C-H bonds
- PMID: 35529210
- PMCID: PMC9070786
- DOI: 10.1039/c9ra04170b
Recent advances in direct trifluoromethylation of olefinic C-H bonds
Abstract
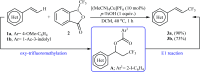
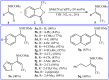
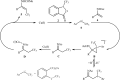
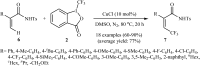
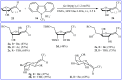
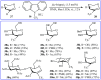
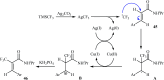
The aim of this review is to provide a comprehensive overview of the direct trifluoromethylation of olefinic C-H bonds with special attention on the mechanistic aspects of the reactions. The review is divided into two major sections. The first focuses exclusively on trifluoromethylation of terminal alkenes, while the second will cover trifluoromethylation of internal alkenes. Literature has been surveyed until the end of April 2019.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




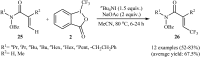
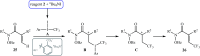
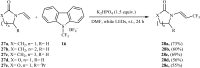


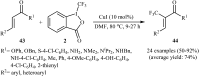

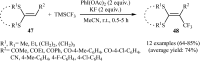




Similar articles
-
Oxidative trifluoromethylation and trifluoromethylthiolation reactions using (trifluoromethyl)trimethylsilane as a nucleophilic CF3 source.Acc Chem Res. 2014 May 20;47(5):1513-22. doi: 10.1021/ar4003202. Epub 2014 Apr 28. Acc Chem Res. 2014. PMID: 24773518 Review.
-
Copper-catalyzed trifluoromethylation of internal olefinic C-H bonds: efficient routes to trifluoromethylated tetrasubstituted olefins and N-heterocycles.Chemistry. 2014 Mar 17;20(12):3439-45. doi: 10.1002/chem.201305069. Chemistry. 2014. PMID: 24677229
-
Recent advances in trifluoromethylation of organic compounds using Umemoto's reagents.Org Biomol Chem. 2014 Sep 14;12(34):6580-9. doi: 10.1039/c4ob00671b. Org Biomol Chem. 2014. PMID: 25011917 Review.
-
Direct C-H trifluoromethylation of di- and trisubstituted alkenes by photoredox catalysis.Beilstein J Org Chem. 2014 May 12;10:1099-106. doi: 10.3762/bjoc.10.108. eCollection 2014. Beilstein J Org Chem. 2014. PMID: 24991259 Free PMC article.
-
Methods for direct C(sp2)-H bonds azidation.RSC Adv. 2019 Aug 13;9(43):25199-25215. doi: 10.1039/c9ra04534a. eCollection 2019 Aug 8. RSC Adv. 2019. PMID: 35528700 Free PMC article. Review.
Cited by
-
Vicinal halo-trifluoromethylation of alkenes.RSC Adv. 2021 Apr 21;11(25):14941-14955. doi: 10.1039/d0ra06872a. eCollection 2021 Apr 21. RSC Adv. 2021. PMID: 35424045 Free PMC article. Review.
-
Synthesis of α-Fluorinated Areneacetates through Photoredox/Copper Dual Catalysis.Org Lett. 2022 May 6;24(17):3194-3198. doi: 10.1021/acs.orglett.2c00969. Epub 2022 Apr 25. Org Lett. 2022. PMID: 35467893 Free PMC article.
-
Hydroxysulfonylation of alkenes: an update.RSC Adv. 2021 Jun 18;11(35):21651-21665. doi: 10.1039/d1ra00513h. eCollection 2021 Jun 15. RSC Adv. 2021. PMID: 35478812 Free PMC article. Review.
-
N-Fluorobenzenesulfonimide: a useful and versatile reagent for the direct fluorination and amination of (hetero)aromatic C-H bonds.RSC Adv. 2020 Apr 29;10(28):16756-16768. doi: 10.1039/d0ra00324g. eCollection 2020 Apr 23. RSC Adv. 2020. PMID: 35498838 Free PMC article. Review.
-
Recent advances in intermolecular 1,2-difunctionalization of alkenes involving trifluoromethylthiolation.RSC Adv. 2021 Jul 13;11(40):24474-24486. doi: 10.1039/d1ra02606b. eCollection 2021 Jul 13. RSC Adv. 2021. PMID: 35481061 Free PMC article. Review.
References
-
- Wang J. Sánchez-Roselló M. Aceña J. L. del Pozo C. Sorochinsky A. E. Fustero S. Soloshonok V. A. Liu H. Chem. Rev. 2014;114:2432–2506. doi: 10.1021/cr4002879. - DOI - PubMed
- Fujiwara T. O'Hagan D. J. Fluorine Chem. 2014;167:16–29. doi: 10.1016/j.jfluchem.2014.06.014. - DOI
- Babudri F. Farinola G. M. Naso F. Ragni R. Chem. Commun. 2007:1003–1022. doi: 10.1039/B611336B. - DOI - PubMed
-
- Kirk K. L. Org. Process Res. Dev. 2008;12:305–321. doi: 10.1021/op700134j. - DOI
-
- Zhu W. Wang J. Wang S. Gu Z. Aceña J. L. Izawa K. Liu H. Soloshonok V. A. J. Fluorine Chem. 2014;167:37–54. doi: 10.1016/j.jfluchem.2014.06.026. - DOI
-
- Zhang C. Adv. Synth. Catal. 2014;356:2895–2906. doi: 10.1002/adsc.201400370. - DOI - PMC - PubMed
- Wang S.-M. Han J.-B. Zhang C.-P. Qin H.-L. Xiao J.-C. Tetrahedron. 2015;71:7949–7976. doi: 10.1016/j.tet.2015.06.056. - DOI
- Lantano B. Torviso M. R. Bonesi S. M. Barata-Vallejo S. Postigo A. Coord. Chem. Rev. 2015;285:76–108. doi: 10.1016/j.ccr.2014.11.004. - DOI
- Guyon H. Chachignon H. Cahard D. Beilstein J. Org. Chem. 2017;13:2764–2799. doi: 10.3762/bjoc.13.272. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

