Zonal rotor centrifugation revisited: new horizons in sorting nanoparticles
- PMID: 35529214
- PMCID: PMC9070787
- DOI: 10.1039/c9ra05140f
Zonal rotor centrifugation revisited: new horizons in sorting nanoparticles
Abstract
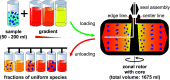
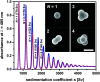
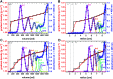
Density gradient centrifugation is an effective method for the isolation and purification of small particles. Hollow rotors capable of hosting density gradients replace the need for centrifuge tubes and therefore allow separations at large scales. So far, zonal rotors have been used for biological separations ranging from the purification of whole cells down to serum proteins. We demonstrate that the high-resolution separation method opens up exciting perspectives apart from biology, namely in sorting mixtures of synthetic nanoparticles. Loading and unloading, while the rotor is spinning, avoids perturbations during acceleration and deceleration periods, and thus makes a vital contribution to sorting accuracy. Nowadays one can synthesize nanoscale particles in a wide variety of compositions and shapes. A prominent example for this are "colloidal molecules" or, generally speaking, defined assemblies of nanoparticles that can appear in varying aggregation numbers. Fractionation of such multimodal colloids plays an essential role with regard to their organization into hierarchical organized superstructures such as films, mesocrystals and metamaterials. Zonal rotor centrifugation was found to be a scalable method of getting "colloidal molecules" properly sorted. It allows access to pure fractions of particle monomers, dimers, and trimers, just as well as to fractions that are essentially rich in particle tetramers. Separations were evaluated by differential centrifugal sedimentation, which provides high-resolution size distributions of the collected nanoparticle fractions. The performance achieved in relation to resolution, zone widths, sorting efficiencies and recovery rates clearly demonstrate that zonal rotor centrifugation provides an excellent solution to the fractionation of nanoparticle mixtures.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Direct Measurement of Sedimentation Coefficient Distributions in Multimodal Nanoparticle Mixtures.Nanomaterials (Basel). 2021 Apr 17;11(4):1027. doi: 10.3390/nano11041027. Nanomaterials (Basel). 2021. PMID: 33920635 Free PMC article.
-
Separation of cell components in the zonal ultracentrifuge.Science. 1962 May 18;136(3516):646-8. doi: 10.1126/science.136.3516.646. Science. 1962. PMID: 13861272
-
Isolation of plasma lipoproteins by zonal ultracentrifugation in the B14 and B15 titanium rotors.J Lipid Res. 1970 Jan;11(1):7-22. J Lipid Res. 1970. PMID: 4189439
-
The resolution of mixtures of viable mammalian cells into homogeneous fractions by zonal centrifugation.J Cell Biol. 1968 Feb;36(2):369-78. doi: 10.1083/jcb.36.2.369. J Cell Biol. 1968. PMID: 5638886 Free PMC article.
-
Ultracentrifugation Techniques for the Ordering of Nanoparticles.Nanomaterials (Basel). 2021 Jan 27;11(2):333. doi: 10.3390/nano11020333. Nanomaterials (Basel). 2021. PMID: 33513966 Free PMC article. Review.
Cited by
-
Optimization of hyphenated asymmetric flow field-flow fractionation for the analysis of silver nanoparticles in aqueous solutions.Anal Bioanal Chem. 2021 Nov;413(27):6889-6904. doi: 10.1007/s00216-021-03647-3. Epub 2021 Sep 19. Anal Bioanal Chem. 2021. PMID: 34537865 Free PMC article.
-
Direct Measurement of Sedimentation Coefficient Distributions in Multimodal Nanoparticle Mixtures.Nanomaterials (Basel). 2021 Apr 17;11(4):1027. doi: 10.3390/nano11041027. Nanomaterials (Basel). 2021. PMID: 33920635 Free PMC article.
-
Soft Sensor Development for Real-Time Process Monitoring of Multidimensional Fractionation in Tubular Centrifuges.Nanomaterials (Basel). 2021 Apr 25;11(5):1114. doi: 10.3390/nano11051114. Nanomaterials (Basel). 2021. PMID: 33923109 Free PMC article.
References
-
- Handbook of Nanomaterials Properties, ed. B. Bhushan, D. Luo, S. R. Schricker, W. Sigmund and S. Zauscher, Springer, Heidelberg, 2014
-
- Kotov N., Nanoparticle Assemblies and Superstructures, CRC Press, Boca Raton, 2005
-
- Thanh N. T. K. Maclean N. Mahiddine S. Chem. Rev. 2014;114:7610. doi: 10.1039/C5CE01014D. - DOI - PubMed
- Pölte J. CrystEngComm. 2015;17:6809. doi: 10.1039/C5CE01014D. - DOI
- Schindler T. Walter J. Peukert W. Segets D. Unruh T. J. Phys. Chem. B. 2015;119:15370. doi: 10.1039/C5CE01014D. - DOI - PubMed
- Carney R. P. Kim J. Y. Qian H. Jin R. Mehenni H. Stellacci F. Bakr O. M. Nat. Commun. 2011;2:335. doi: 10.1039/C5CE01014D. - DOI - PMC - PubMed
-
- Mostafa S. Behafarid F. Croy J. R. Ono L. K. Li L. Yang J. C. Frenkel A. I. Cuenya B. R. J. Am. Chem. Soc. 2010;132:15714. doi: 10.1039/B507240A. - DOI - PubMed
- Orendorff C. J. Sau T. K. Murphy C. J. Small. 2006;2:636. doi: 10.1039/B507240A. - DOI - PubMed
- Sun H.-L. Shi H. Zhao F. Qi L. Gao S. Chem. Commun. 2005:4339. doi: 10.1039/B507240A. - DOI - PubMed
- Gómez-Ramírez A. López-López M. T. Durán J. D. G. González-Caballero F. Soft Matter. 2009;5:3888. doi: 10.1039/B507240A. - DOI - PubMed
- Baalousha M. Lead J. R. Nat. Nanotechnol. 2013;8:308. doi: 10.1039/B507240A. - DOI - PubMed
LinkOut - more resources
Full Text Sources

