Circulating inflammatory monocytes oppose microglia and contribute to cone cell death in retinitis pigmentosa
- PMID: 35529318
- PMCID: PMC9075747
- DOI: 10.1093/pnasnexus/pgac003
Circulating inflammatory monocytes oppose microglia and contribute to cone cell death in retinitis pigmentosa
Abstract
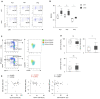
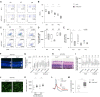
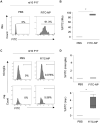
Retinitis pigmentosa (RP) is an intractable inherited disease that primarily affects the rods through gene mutations followed by secondary cone degeneration. This cone-related dysfunction can lead to impairment of daily life activities, and ultimately blindness in patients with RP. Paradoxically, microglial neuroinflammation contributes to both protection against and progression of RP, but it is unclear which population(s) - tissue-resident microglia and/or peripheral monocyte-derived macrophages (mφ) - are implicated in the progression of the disease. Here we show that circulating blood inflammatory monocytes (IMo) are key effector cells that mediate cone cell death in RP. Attenuation of IMo and peripherally engrafted mφ by Ccl2 deficiency or immune modulation via intravenous nano-particle treatment suppressed cone cell death in rd10 mice, an animal model of RP. In contrast, the depletion of resident microglia by a colony-stimulating factor 1 receptor inhibitor exacerbated cone cell death in the same model. In human patients with RP, IMo was increased and correlated with disease progression. These results suggest that peripheral IMo is a potential target to delay cone cell death and prevent blindness in RP.
Keywords: Nanomedicine; Neuroinflammation; Peripheral monocyte.
Conflict of interest statement
Conflict of interest statement: The authors have declared that no conflict of interest exists.
Figures
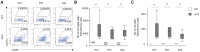

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

