Transition metal-free one-pot synthesis of substituted pyrroles by employing aza-Wittig reaction
- PMID: 35529397
- PMCID: PMC9072206
- DOI: 10.1039/c9ra06778g
Transition metal-free one-pot synthesis of substituted pyrroles by employing aza-Wittig reaction
Abstract
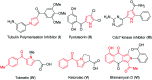
A mild and metal-free one-pot synthetic strategy has been developed for the construction of substituted pyrroles by employing aza-Wittig reaction from a unique and unexplored combination of chromones and phenacyl azides. This method does not compromise the diverse substitutions on both the phenacyl azides and chromones. The merits of this method are wide substrate scope, easy functionalization, short reaction time, operationally simple, and higher yields. Moreover, this method is amenable for the generation of a library of key pyrrole building blocks.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
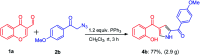

References
-
- Estevez V. Villacampa M. Menendez J. C. Chem. Soc. Rev. 2010;39:4402. doi: 10.1039/B917644F. - DOI - PubMed
- Estevez V. Villacampa M. Menendez J. C. Chem. Soc. Rev. 2014;43:4633. doi: 10.1039/C3CS60015G. - DOI - PubMed
- Fan H. Peng J. Hamann M. T. Hu J. F. Chem. Rev. 2008;108:264. doi: 10.1021/cr078199m. - DOI - PMC - PubMed
- Hall A. Atkinson S. Brown S. H. Chessell I. P. Chowdhury A. Giblin G. M. P. Goldsmith P. Healy M. P. Jandu K. S. Johnson M. R. Michel A. D. Naylor A. Sweeting J. A. Bioorg. Med. Chem. Lett. 2007;17:1200. doi: 10.1016/j.bmcl.2006.12.021. - DOI - PubMed
- Galliford C. V. Scheidt K. A. Angew. Chem., Int. Ed. 2007;46:8748. doi: 10.1002/anie.200701342. - DOI - PubMed
-
- Coen L. M. D. Heugebaert T. S. A. Garcia D. Stevens C. V. Chem. Rev. 2016;116:80. doi: 10.1021/acs.chemrev.5b00483. - DOI - PubMed
- Blunt J. W. Copp B. R. Keyzers R. A. Munroa M. H. G. Prinsep M. R. Nat. Prod. Rep. 2014;31:160. doi: 10.1039/C3NP70117D. - DOI - PubMed
- Mohamed M. S. Fathallah S. S. Mini-Rev. Org. Chem. 2014;11:477. doi: 10.2174/1570193X113106660018. - DOI
- Gholap S. S. Eur. J. Med. Chem. 2016;110:13. doi: 10.1016/j.ejmech.2015.12.017. - DOI - PubMed
- Takase M. Narita T. Fujita W. Asano M. S. Nishinaga T. Benten H. Yoza K. Mullen K. J. Am. Chem. Soc. 2013;135:8031. doi: 10.1021/ja402371f. - DOI - PubMed
- Imbri D. Tauber J. Opatz T. Mar. Drugs. 2014;12:6142. doi: 10.3390/md12126142. - DOI - PMC - PubMed
-
- Toube T. P., Trofimov B. A., Cirrincione G., Almerico A. M., Aiello E., Dattolo G., McNab H. and Monahan L. C., in Chemistry of Heterocyclic Compounds: Pyrroles, Part II, The Synthesis, Reactivity and Physical Properties of Substituted Pyrroles, ed. R. A. Jones, John Wiley & Sons Inc., New York, 2008, vol. 48, p. 1
- Pelkey E. T., in Progress in Heterocyclic Chemistry, ed. G. W. Gribble and J. A. Joule, Elsevier, S. A., 2005, vol. 17, p. 109
LinkOut - more resources
Full Text Sources

