Theabrownins Produced via Chemical Oxidation of Tea Polyphenols Inhibit Human Lung Cancer Cells in vivo and in vitro by Suppressing the PI3K/AKT/mTOR Pathway Activation and Promoting Autophagy
- PMID: 35529455
- PMCID: PMC9070389
- DOI: 10.3389/fnut.2022.858261
Theabrownins Produced via Chemical Oxidation of Tea Polyphenols Inhibit Human Lung Cancer Cells in vivo and in vitro by Suppressing the PI3K/AKT/mTOR Pathway Activation and Promoting Autophagy
Abstract
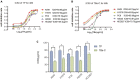
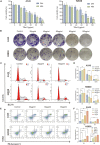
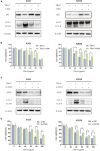
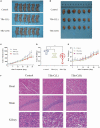
During the fermentation of dark tea, theabrownins (TBs), carbohydrates, and other substances get irreversibly complex. Recent research on the biological activity of TBs is not based on free TBs. In the present study, some brown polyphenol oxidized polymers, the generalized TBs (TBs-C), were prepared via alkali oxidation from tea polyphenols (TP). We also investigated the inhibitory mechanism of TBs-C on non-small-cell-lung cancer (NSCLC). TBs-C demonstrated a stronger inhibition than TP on the NSCLC cell lines A549, H2030, HCC827, H1975, and PC9. Next, A549 and H2030 cell lines were selected as subjects to explore this mechanism. TBs-C was found to inhibit proliferation, promote apoptosis, and induce G1 cell-cycle arrest in the cells. In addition, TBs-C increased autophagic flux, which in turn promoted the death of lung cancer cells. Moreover, TBs-C suppressed the PI3K/AKT/mTOR pathway activation, promoted autophagy, and increased the expression of p21 downstream of AKT, which resulted in G1 cell-cycle arrest. In xenotransplanted NSCLC nude mice derived from A549 cells, TBs-C could significantly suppress tumor growth by inhibiting the PI3K/AKT/mTOR pathway without causing hepatotoxicity, brain toxicity, or nephrotoxicity. We believe that our present findings would facilitate advancement in the research and industrialization of TBs.
Keywords: chemical oxidation; human lung cancer; in vivo and in vitro; tea polyphenols; theabrownins.
Copyright © 2022 Wang, Yuan, Wang, Wang, Zou, Zhang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Physicochemical and Colon Cancer Cell Inhibitory Properties of Theabrownins Prepared by Weak Alkali Oxidation of Tea Polyphenols.Plant Foods Hum Nutr. 2022 Sep;77(3):405-411. doi: 10.1007/s11130-022-00988-x. Epub 2022 Jul 7. Plant Foods Hum Nutr. 2022. PMID: 35794451
-
Characterization of Theabrownins Prepared From Tea Polyphenols by Enzymatic and Chemical Oxidation and Their Inhibitory Effect on Colon Cancer Cells.Front Nutr. 2022 Mar 15;9:849728. doi: 10.3389/fnut.2022.849728. eCollection 2022. Front Nutr. 2022. PMID: 35369086 Free PMC article.
-
Physicochemical stability and antibacterial mechanism of theabrownins prepared from tea polyphenols catalyzed by polyphenol oxidase and peroxidase.Food Sci Biotechnol. 2023 May 30;33(1):47-61. doi: 10.1007/s10068-023-01341-x. eCollection 2024 Jan. Food Sci Biotechnol. 2023. PMID: 38186623 Free PMC article.
-
Luteoloside induces G0/G1 arrest and pro-death autophagy through the ROS-mediated AKT/mTOR/p70S6K signalling pathway in human non-small cell lung cancer cell lines.Biochem Biophys Res Commun. 2017 Dec 9;494(1-2):263-269. doi: 10.1016/j.bbrc.2017.10.042. Epub 2017 Oct 9. Biochem Biophys Res Commun. 2017. PMID: 29024631
-
State-of-the-art review of theabrownins: from preparation, structural characterization to health-promoting benefits.Crit Rev Food Sci Nutr. 2024 Nov;64(31):11321-11340. doi: 10.1080/10408398.2023.2236701. Epub 2023 Aug 16. Crit Rev Food Sci Nutr. 2024. PMID: 37584203 Review.
Cited by
-
Mechanism of the combined action of green tea polyphenols and concurrent radiochemotherapy in regulating GSK-3β to treat non-small cell lung cancer through the Wnt∕β-catenin pathway.Rom J Morphol Embryol. 2024 Jul-Sep;65(3):499-505. doi: 10.47162/RJME.65.3.12. Rom J Morphol Embryol. 2024. PMID: 39529343 Free PMC article.
-
Differential regulation of STING expression and cisplatin sensitivity by autophagy in non-small cell lung cancer cells.Med Oncol. 2025 May 30;42(7):227. doi: 10.1007/s12032-025-02786-2. Med Oncol. 2025. PMID: 40445433 Free PMC article.
-
Dietary Polyphenols against Oxidative Stress in Head and Neck Cancer: What's New, What's Next.J Cancer. 2024 Jan 1;15(2):293-308. doi: 10.7150/jca.90545. eCollection 2024. J Cancer. 2024. PMID: 38169656 Free PMC article. Review.
-
Preventive and therapeutic effects of green tea on lung cancer: a narrative review of evidence from clinical and basic research.J Thorac Dis. 2022 Dec;14(12):5029-5038. doi: 10.21037/jtd-22-1791. J Thorac Dis. 2022. PMID: 36647481 Free PMC article. Review.
-
Theabrownin Alleviates Colorectal Tumorigenesis in Murine AOM/DSS Model via PI3K/Akt/mTOR Pathway Suppression and Gut Microbiota Modulation.Antioxidants (Basel). 2022 Aug 30;11(9):1716. doi: 10.3390/antiox11091716. Antioxidants (Basel). 2022. PMID: 36139789 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

