Immune Response to Third Dose BNT162b2 COVID-19 Vaccine Among Kidney Transplant Recipients-A Prospective Study
- PMID: 35529596
- PMCID: PMC9068869
- DOI: 10.3389/ti.2022.10204
Immune Response to Third Dose BNT162b2 COVID-19 Vaccine Among Kidney Transplant Recipients-A Prospective Study
Abstract
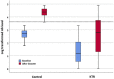
Immune response to two SARS-CoV-2 mRNA vaccine doses among kidney transplant recipients (KTRs) is limited. We aimed to evaluate humoral and cellular response to a third BNT162b2 dose. In this prospective study, 190 KTRs were evaluated before and ∼3 weeks after the third vaccine dose. The primary outcomes were anti-spike antibody level >4160 AU/ml (neutralization-associated cutoff) and any seropositivity. Univariate and multivariate analyses were conducted to identify variables associated with antibody response. T-cell response was evaluated in a subset of participants. Results were compared to a control group of 56 healthcare workers. Among KTRs, we found a seropositivity rate of 70% (133/190) after the third dose (37%, 70/190, after the second vaccine dose); and 27% (52/190) achieved levels above 4160 AU/ml after the third dose, compared to 93% of controls. Variables associated with antibody response included higher antibody levels after the second dose (odds ratio [OR] 30.8 per log AU/ml, 95% confidence interval [CI]11-86.4, p < 0.001); and discontinuation of antimetabolite prior to vaccination (OR 9.1,95% CI 1.8-46.5, p = 0.008). T-cell response was demonstrated in 13% (7/53). In conclusion, third dose BNT162b2 improved immune response among KTRs, however 30% still remained seronegative. Pre-vaccination temporary immunosuppression reduction improved antibody response.
Keywords: COVID-19 vaccine; antibody response; cellular response; immunosuppression reduction; kidney transplant recipients.
Copyright © 2022 Yahav, Rahamimov, Mashraki, Ben-Dor, Steinmetz, Agur, Zingerman, Herman-Edelstein, Lichtenberg, Ben-Zvi, Bar-Haim, Cohen, Rotem, Elia, Margalit and Zvi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- American Society of Transplantation. COVID-19: Vaccine FAQ Sheet [Internet]. United States: American Society of Transplantation; (2020). Available from: https://www.myast.org/covid-19-vaccine-faq-sheet (cited Sep 26, 2021).
-
- Crespo M, Barrilado-Jackson A, Padilla E, Eguía J, Echeverria-Esnal D, Cao H, et al. Negative Immune Responses to Two-Dose mRNA COVID-19 Vaccines in Renal Allograft Recipients Assessed with Simple Antibody and Interferon Gamma Release Assay Cellular Monitoring. Am J Transpl (2022) 22:786–800. 10.1111/ajt.16854 - DOI - PMC - PubMed
-
- Stumpf J, Siepmann T, Lindner T, Karger C, Schwöbel J, Anders L, et al. Humoral and Cellular Immunity to SARS-CoV-2 Vaccination in Renal Transplant versus Dialysis Patients: A Prospective, Multicenter Observational Study Using mRNA-1273 or BNT162b2 mRNA Vaccine. The Lancet Reg Health - Europe (2021) 9:100178. 10.1016/j.lanepe.2021.100178 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

