Retracted Article: Gm5820, an antisense RNA of FGF1, suppresses FGF1 expression at the posttranscriptional level to inactivate the ERK/STAT3 pathway and alleviates neuropathic pain in mice
- PMID: 35529622
- PMCID: PMC9071159
- DOI: 10.1039/c9ra03791h
Retracted Article: Gm5820, an antisense RNA of FGF1, suppresses FGF1 expression at the posttranscriptional level to inactivate the ERK/STAT3 pathway and alleviates neuropathic pain in mice
Retraction in
-
Retraction: Gm5820, an antisense RNA of FGF1, suppresses FGF1 expression at the posttranscriptional level to inactivate the ERK/STAT3 pathway and alleviates neuropathic pain in mice.RSC Adv. 2021 Jan 26;11(9):5024. doi: 10.1039/d1ra90034j. eCollection 2021 Jan 25. RSC Adv. 2021. PMID: 35686055 Free PMC article.
Abstract
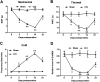
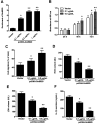
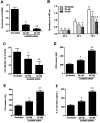
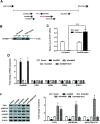
Emerging evidence reveals that lncRNAs play important roles in various pathological processes, but precious little indicates their regulatory role in neuropathic pain. In this study, we performed unbiased whole transcriptome profiling in dorsal root ganglions (DRGs) from mice with sham operation and mice with chronic constriction injury (CCI). Gm5820 was one of the most downregulated RNA transcripts in CCI neuropathic pain model mice. Then, a pcDNA-Gm5820 expression vector was constructed and administered into CCI mice through intrathecal injection. The results showed that upregulation of Gm5820 alleviated mouse mechanical allodynia and thermal/cold hyperalgesia, and reduced the accumulation of inflammatory cytokines and ROS in the DRG tissue. Moreover, different concentrations of pcDNA-Gm5820 expression vector and Gm5820 siRNA were respectively transfected into primary DRG neurons from 4th to 6th lumbar vertebra (L4-L6). We found that Gm5820 overexpression improved cell viability and migration, and reduced the production of ROS, LDH and IL-1β. In contrast, Gm5820 knockdown had the opposite effects. Furthermore, RNA pull-down assays with FGF1 and Gm5820 cDNA probes both demonstrated that FGF1 mRNA and Gm5820 directly bound to each other. Moreover, Gm5820 negatively regulated the stability of FGF1 mRNA. Gm5820 suppressed the expression of FGF1 at the post-transcriptional level and negatively regulated the activation of ERK1/2-mediated STAT3, a critical contributor in neuropathic pain. In conclusion, Gm5820 directly binds to FGF1 mRNA and suppresses FGF1 expression at the posttranscriptional level, it functions as a negative regulator in the activation of the ERK/STAT3 pathway, and upregulation of Gm5820 alleviates neuropathic pain in CCI mice.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declared that they have no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

