A molecular electron density theory study of the mechanism, chemo- and stereoselectivity of the epoxidation reaction of R-carvone with peracetic acid
- PMID: 35529642
- PMCID: PMC9071017
- DOI: 10.1039/c9ra05309c
A molecular electron density theory study of the mechanism, chemo- and stereoselectivity of the epoxidation reaction of R-carvone with peracetic acid
Abstract
The epoxidation reaction of R-carvone 8 with peracetic acid 9 has been studied within the molecular electron density theory at the B3LYP/6-311(d,p) computational level. The chemo- and stereoisomeric reaction paths involving the two C-C double bonds of R-carvone 8 have been studied. DFT calculations account for the high chemoselectivity involving the C-C double bond of the isopropenyl group and the low diastereoselectivity, in complete agreement with the experimental outcomes. The Baeyer-Villiger reaction involving the carbonyl group of R-carvone 8 has also been analysed. A bonding evolution theory analysis of the epoxidation reaction shows the complexity of the bonding changes taking place along this reaction. Formation of the oxirane ring takes place asynchronously at the end of the reaction by attack of anionic oxygen on the two carbons of the isopropenyl C-C double bond.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
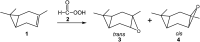









Similar articles
-
Chemical reactivities and molecular docking studies of parthenolide with the main protease of HEP-G2 and SARS-CoV-2.J Mol Struct. 2021 Nov 5;1243:130705. doi: 10.1016/j.molstruc.2021.130705. Epub 2021 May 19. J Mol Struct. 2021. PMID: 34031619 Free PMC article.
-
A practical deca-gram scale ring expansion of (R)-(-)-carvone to (R)-(+)-3-methyl-6-isopropenyl-cyclohept-3-enone-1.Org Biomol Chem. 2015 Jul 28;13(28):7633-42. doi: 10.1039/c5ob00525f. Epub 2015 May 22. Org Biomol Chem. 2015. PMID: 25997609
-
MEDT analysis of mechanism and selectivities in non-catalyzed and lewis acid-catalyzed diels-alder reactions between R-carvone and isoprene.Sci Rep. 2024 Jul 22;14(1):16827. doi: 10.1038/s41598-024-67351-9. Sci Rep. 2024. PMID: 39039149 Free PMC article.
-
Olefin epoxidation by molybdenum peroxo compound: molecular mechanism characterized by the electron localization function and catastrophe theory.J Phys Chem A. 2011 Feb 3;115(4):514-22. doi: 10.1021/jp108440f. Epub 2010 Dec 29. J Phys Chem A. 2011. PMID: 21190350
-
A molecular electron density theory study of the [3 + 2] cycloaddition reaction of nitrones with ketenes.Org Biomol Chem. 2017 Feb 21;15(7):1618-1627. doi: 10.1039/c6ob02768g. Epub 2017 Jan 25. Org Biomol Chem. 2017. PMID: 28120980
Cited by
-
Description of Chiral Complexes within Functional-Group Symmetry-Adapted Perturbation Theory-The Case of (S/R)-Carvone with Derivatives of (-)-Menthol.J Phys Chem A. 2020 Sep 24;124(38):7735-7748. doi: 10.1021/acs.jpca.0c06266. Epub 2020 Sep 14. J Phys Chem A. 2020. PMID: 32856904 Free PMC article.
-
Chemical reactivities and molecular docking studies of parthenolide with the main protease of HEP-G2 and SARS-CoV-2.J Mol Struct. 2021 Nov 5;1243:130705. doi: 10.1016/j.molstruc.2021.130705. Epub 2021 May 19. J Mol Struct. 2021. PMID: 34031619 Free PMC article.
-
Evaluation of inhibitive corrosion potential of symmetrical hydrazine derivatives containing nitrophenyl moiety in 1M HCl for C38 steel: experimental and theoretical studies.Heliyon. 2022 Mar 15;8(3):e09087. doi: 10.1016/j.heliyon.2022.e09087. eCollection 2022 Mar. Heliyon. 2022. PMID: 35846476 Free PMC article.
References
LinkOut - more resources
Full Text Sources

