Current perspectives on the beneficial effects of soybean isoflavones and their metabolites on plants
- PMID: 35529690
- PMCID: PMC9033921
- DOI: 10.1007/s10068-022-01070-7
Current perspectives on the beneficial effects of soybean isoflavones and their metabolites on plants
Abstract
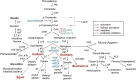
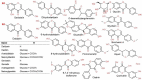
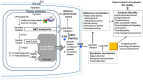
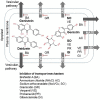
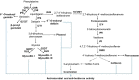
Soybeans have traditionally been a staple part of the human diet being highly rich in protein and lipid content. In an addition to the high nutritional components, soybeans have several functional components, like isoflavones, saponins, lecithin, and oligosaccharides. Soybeans emerge as a healthy functional food option. Isoflavones are most notable functional component of soybeans, exhibiting antioxidant activity while preventing plant-related diseases (e.g., antimicrobial and antiherbivore activities) and having positive effects on the life quality of plants. Isoflavones are thus sometimes referred to as phytochemicals. The latest research trends evince substantial interest in the biological efficacy of isoflavones in the human body as well as in plants and their related mechanisms. However, there is little information on the relationship between isoflavones and plants than beneficial human effects. This review discusses what is known about the physiological communication (transport and secretion) between isoflavones and plants, especially in soybeans.
Keywords: Environmental adaptation; Plant; Rhizobium; Soybean-derived isoflavone; Symbiotic interaction.
© The Korean Society of Food Science and Technology 2022.
Figures
Similar articles
-
Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans.Antioxidants (Basel). 2021 Jun 30;10(7):1064. doi: 10.3390/antiox10071064. Antioxidants (Basel). 2021. PMID: 34209224 Free PMC article. Review.
-
pH-adjusted solvent extraction and reversed-phase HPLC quantification of isoflavones from soybean (Glycine max (L.) Merr.).J Food Sci. 2020 Mar;85(3):673-681. doi: 10.1111/1750-3841.15051. Epub 2020 Feb 20. J Food Sci. 2020. PMID: 32078761
-
Biochemistry and use of soybean isoflavones in functional food development.Crit Rev Food Sci Nutr. 2020;60(12):2098-2112. doi: 10.1080/10408398.2019.1630598. Epub 2019 Jul 5. Crit Rev Food Sci Nutr. 2020. PMID: 31272191 Review.
-
Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing.J Nutr. 1995 Mar;125(3 Suppl):581S-588S. doi: 10.1093/jn/125.3_Suppl.581S. J Nutr. 1995. PMID: 7884537 Review.
-
MATE-Type Proteins Are Responsible for Isoflavone Transportation and Accumulation in Soybean Seeds.Int J Mol Sci. 2021 Nov 6;22(21):12017. doi: 10.3390/ijms222112017. Int J Mol Sci. 2021. PMID: 34769445 Free PMC article.
Cited by
-
A new biostimulant derived from soybean by-products enhances plant tolerance to abiotic stress triggered by ozone.BMC Plant Biol. 2024 Jun 19;24(1):580. doi: 10.1186/s12870-024-05290-3. BMC Plant Biol. 2024. PMID: 38890606 Free PMC article.
-
Identification of South African Plant-Based Bioactive Compounds as Potential Inhibitors against the SARS-CoV-2 Receptor.Pharmaceuticals (Basel). 2024 Jun 22;17(7):821. doi: 10.3390/ph17070821. Pharmaceuticals (Basel). 2024. PMID: 39065672 Free PMC article.
-
Functional characterization and structural basis of a reversible glycosyltransferase involves in plant chemical defence.Plant Biotechnol J. 2023 Dec;21(12):2611-2624. doi: 10.1111/pbi.14157. Epub 2023 Aug 15. Plant Biotechnol J. 2023. PMID: 37581303 Free PMC article.
-
Phytoestrogens Present in Follicular Fluid and Urine Are Positively Associated with IVF Outcomes following Single Euploid Embryo Transfer.Int J Mol Sci. 2023 Jun 29;24(13):10852. doi: 10.3390/ijms241310852. Int J Mol Sci. 2023. PMID: 37446033 Free PMC article.
-
Perspective on the Coevolutionary Role of Host and Gut Microbiota in Polyphenol Health Effects: Metabotypes and Precision Health.Mol Nutr Food Res. 2024 Nov;68(22):e2400526. doi: 10.1002/mnfr.202400526. Epub 2024 Nov 13. Mol Nutr Food Res. 2024. PMID: 39538982 Free PMC article. Review.
References
-
- Ahemad M, Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. Journal of King Saud University-Science. 2014;26:1–20. doi: 10.1016/j.jksus.2013.05.001. - DOI
-
- Ahkami AH, Allen White R, Handakumbura PP, Jansson C. Rhizosphere engineering: Enhancing sustainable plant ecosystem productivity. Rhizosphere. 2017;3:233–243. doi: 10.1016/j.rhisph.2017.04.012. - DOI
Publication types
LinkOut - more resources
Full Text Sources
